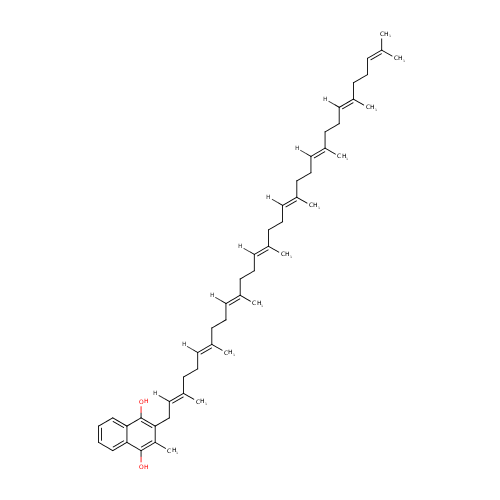
Menaquinol 8 (PAMDB001653)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001653 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Menaquinol 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Menaquinol 8 is a polyprenylhydroquinone having a an octaprenyl moiety at position 2 and a methyl group at position 3. It is a substrate for Dimethyl sulfoxide reductase (dmsA). This enzyme catalyzes the reduction of dimethyl sulfoxide (DMSO) to dimethyl sulfide (DMS) using the following reaction: Dimethylsulfide + menaquinone + H2O = dimethylsulfoxide + menaquinol. DMSO reductase serves as the terminal reductase under anaerobic conditions, with DMSO being the terminal electron acceptor. Terminal reductase during anaerobic growth on various sulfoxides and N-oxide compounds. This enzyme allows P. aeruginosa to grow anaerobically on DMSO as respiratory oxidant. Menaquinol 8 is generated by Ubiquinone/menaquinone biosynthesis methyltransferase (ubiE). This enzyme is required for the conversion of demethylmenaquinone (DMKH2) to menaquinone (MKH2) and has the following catalytic activity: A demethylmenaquinone + S-adenosyl-L-methionine = a menaquinol + S-adenosyl-L-homocysteine. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C51H74O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 719.1321 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 718.568881612 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | OIEZRVBFVPGODT-WQWYCSGDSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C51H74O2/c1-38(2)20-13-21-39(3)22-14-23-40(4)24-15-25-41(5)26-16-27-42(6)28-17-29-43(7)30-18-31-44(8)32-19-33-45(9)36-37-47-46(10)50(52)48-34-11-12-35-49(48)51(47)53/h11-12,20,22,24,26,28,30,32,34-36,52-53H,13-19,21,23,25,27,29,31,33,37H2,1-10H3/b39-22+,40-24+,41-26+,42-28+,43-30+,44-32+,45-36+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-methyl-3-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]naphthalene-1,4-diol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | menaquinol-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC1=C(O)C2=CC=CC=C2C(O)=C1C)=C(\C)CCC=C(C)C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as polyprenyl quinols. These are compounds containing a polyisoprene chain attached to a quinol(hydroquinone) at the second ring position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Quinone and hydroquinone lipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Polyprenyl quinols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homopolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Hydrogen ion + Menaquinol 8 + Trimethylamine N-Oxide > Water + Menaquinone 8 + Trimethylamine 2 Hydrogen ion + Hydrogen (gas) + Menaquinone 8 > Menaquinol 8 +2 Hydrogen ion 2 Hydrogen ion + Menaquinol 8 + Nitrate > Water + Menaquinone 8 + Nitrite +2 Hydrogen ion 2 Hydrogen ion + Menaquinone 8 + Formic acid > Menaquinol 8 + Carbon dioxide + Hydrogen ion Dimethyl sulfoxide + Menaquinol 8 > Dimethyl sulfide + Water + Menaquinone 8 Menaquinol 8 + Nitrate > Menaquinone 8 + Water + Nitrite 2 Hydrogen ion + Menaquinol 8 + Oxygen > Water + Menaquinone 8 +2 Hydrogen ion Menaquinol 8 + Selenocystathionine > Water + Menaquinone 8 + Selenite Glycerol 3-phosphate + Menaquinone 8 > Dihydroxyacetone phosphate + Menaquinol 8 4 Hydrogen ion + Menaquinone 8 + NADH > Menaquinol 8 + NAD +3 Hydrogen ion Glycolic acid + Menaquinone 8 > Glyoxylic acid + Menaquinol 8 L-Lactic acid + Menaquinone 8 > Menaquinol 8 + Pyruvic acid Menaquinone 8 + periplasmic protein disulfide isomerase I (reduced) > Menaquinol 8 + periplasmic protein disulfide isomerase I (oxidized) 3 Menaquinol 8 + 2 Hydrogen ion + Nitrite >3 Menaquinone 8 +2 Water + Ammonium Fumaric acid + Menaquinol 8 > Menaquinone 8 + Succinic acid 4,5-Dihydroorotic acid + Menaquinone 8 > Menaquinol 8 + Orotic acid Hydrogen ion + Menaquinone 8 + NADH > Menaquinol 8 + NAD L-Malic acid + Menaquinone 8 > Menaquinol 8 + Oxalacetic acid L-Aspartic acid + Menaquinone 8 > Hydrogen ion + Iminoaspartic acid + Menaquinol 8 Hydrogen ion + Menaquinone 8 + NADPH > Menaquinol 8 + NADP Menaquinol 8 + 2 Oxygen >2 Hydrogen ion + Menaquinone 8 +2 Superoxide anion 2-Demethylmenaquinol 8 + S-Adenosylmethionine > S-Adenosylhomocysteine + Hydrogen ion + Menaquinol 8 Glycerol 3-phosphate + menaquinone-8 > Menaquinol 8 + Dihydroxyacetone phosphate More...2-Demethylmenaquinol 8 + S-adenosyl-L-methionine > Hydrogen ion + S-Adenosylhomocysteine + Menaquinol 8 NADH + 4 Hydrogen ion + 2 Hydrogen ion + menaquinone-8 NAD + Hydrogen ion + Menaquinol 8 + Electron +4 Hydrogen ion Trimethylamine N-Oxide + 3 Hydrogen ion + Menaquinol 8 + 2 Electron > Trimethylamine + Water +2 Hydrogen ion + menaquinone-8 Hydrogen ion + Electron + 2 Hydrogen ion + menaquinone-8 > Menaquinol 8 + Hydrogen ion Formic acid + menaquinone-8 + Electron + Hydrogen ion > Carbon dioxide + Hydrogen ion + Menaquinol 8 Menaquinol 8 + Dimethyl sulfoxide + 2 Hydrogen ion + 2 Electron > menaquinone-8 + Dimethyl sulfide + Water +2 Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidation-reduction process
- Specific function:
- Transfer of electrons from NADH to the respiratory chain. The immediate electron acceptor for the enzyme is believed to be ubiquinone. Does not couple the redox reaction to proton translocation
- Gene Name:
- ndh
- Locus Tag:
- PA4538
- Molecular weight:
- 47.4 kDa
Reactions
| NADH + acceptor = NAD(+) + reduced acceptor. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The alpha chain is the actual site of nitrate reduction
- Gene Name:
- narG
- Locus Tag:
- PA3875
- Molecular weight:
- 141 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in catalytic activity
- Specific function:
- (S)-dihydroorotate + a quinone = orotate + a quinol
- Gene Name:
- pyrD
- Locus Tag:
- PA3050
- Molecular weight:
- 36.1 kDa
Reactions
| (S)-dihydroorotate + a quinone = orotate + a quinol. |
- General function:
- Involved in electron carrier activity
- Specific function:
- Formate dehydrogenase allows Pseudomonas aeruginosa to use formate as major electron donor during anaerobic respiration, when nitrate is used as electron acceptor. The beta chain is an electron transfer unit containing 4 cysteine clusters involved in the formation of iron-sulfur centers. Electrons are transferred from the gamma chain to the molybdenum cofactor of the alpha subunit
- Gene Name:
- fdnH
- Locus Tag:
- PA4811
- Molecular weight:
- 33.8 kDa
- General function:
- Involved in respiratory electron transport chain
- Specific function:
- Formate dehydrogenase allows Pseudomonas aeruginosa to use formate as major electron donor during anaerobic respiration, when nitrate is used as electron acceptor. Subunit gamma is the cytochrome b556(FDN) component of the formate dehydrogenase
- Gene Name:
- fdnI
- Locus Tag:
- PA4810
- Molecular weight:
- 23.9 kDa
- General function:
- Involved in catalytic activity
- Specific function:
- Specific function unknown
- Gene Name:
- glcD
- Locus Tag:
- PA5355
- Molecular weight:
- 53.7 kDa
- General function:
- Involved in oxidoreductase activity, acting on NADH or NADPH
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoA
- Locus Tag:
- PA2637
- Molecular weight:
- 15 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in NADH dehydrogenase (ubiquinone) activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoB
- Locus Tag:
- PA2638
- Molecular weight:
- 25.4 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoE
- Locus Tag:
- PA2640
- Molecular weight:
- 18.1 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in oxidation-reduction process
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient. This subunit may bind ubiquinone
- Gene Name:
- nuoH
- Locus Tag:
- PA2643
- Molecular weight:
- 36.7 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in electron carrier activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoI
- Locus Tag:
- PA2644
- Molecular weight:
- 20.6 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in NADH dehydrogenase (ubiquinone) activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoJ
- Locus Tag:
- PA2645
- Molecular weight:
- 17.6 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in oxidoreductase activity, acting on NADH or NADPH
- Specific function:
- There are 2 NADH dehydrogenases in Pseudomonas aeruginosa, however only this complex is able to use dNADH (reduced nicotinamide hypoxanthine dinucleotide, deamino-NADH) and dNADH-DB (dimethoxy- 5-methyl-6-decyl-1,4-benzoquinone) as substrates
- Gene Name:
- nuoK
- Locus Tag:
- PA2646
- Molecular weight:
- 11 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in NADH dehydrogenase (ubiquinone) activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoM
- Locus Tag:
- PA2648
- Molecular weight:
- 55.7 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in NADH dehydrogenase (ubiquinone) activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoN
- Locus Tag:
- PA2649
- Molecular weight:
- 51.7 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in electron carrier activity
- Specific function:
- Catalyzes the oxidation of L-aspartate to iminoaspartate
- Gene Name:
- nadB
- Locus Tag:
- PA0761
- Molecular weight:
- 60 kDa
Reactions
| L-aspartate + O(2) = iminosuccinate + H(2)O(2). |
- General function:
- Involved in iron-sulfur cluster binding
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The beta chain is an electron transfer unit containing four cysteine clusters involved in the formation of iron-sulfur centers. Electrons are transferred from the gamma chain to the molybdenum cofactor of the alpha subunit
- Gene Name:
- narH
- Locus Tag:
- PA3874
- Molecular weight:
- 58.1 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in nitrate reductase activity
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The gamma chain is a membrane-embedded heme-iron unit resembling cytochrome b, which transfers electrons from quinones to the beta subunit
- Gene Name:
- narI
- Locus Tag:
- PA3872
- Molecular weight:
- 25 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in formate dehydrogenase (NAD+) activity
- Specific function:
- Formate dehydrogenase allows Pseudomonas aeruginosa to use formate as major electron donor during anaerobic respiration, when nitrate is used as electron acceptor. The alpha subunit forms the active site
- Gene Name:
- fdnG
- Locus Tag:
- PA4812
- Molecular weight:
- 104.7 kDa
Reactions
| Formate + NAD(+) = CO(2) + NADH. |
- General function:
- Involved in NADH dehydrogenase (ubiquinone) activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoF
- Locus Tag:
- PA2641
- Molecular weight:
- 48.7 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in catalytic activity
- Specific function:
- (S)-lactate + 2 ferricytochrome c = pyruvate + 2 ferrocytochrome c + 2 H(+)
- Gene Name:
- lldD
- Locus Tag:
- PA4771
- Molecular weight:
- 41.1 kDa
Reactions
| (S)-lactate + 2 ferricytochrome c = pyruvate + 2 ferrocytochrome c + 2 H(+). |
- General function:
- Involved in oxidoreductase activity, acting on NADH or NADPH
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoC
- Locus Tag:
- PA2639
- Molecular weight:
- 68.3 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in electron carrier activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoG
- Locus Tag:
- PA2642
- Molecular weight:
- 99 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in NADH dehydrogenase (ubiquinone) activity
- Specific function:
- NDH-1 shuttles electrons from NADH, via FMN and iron- sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is believed to be ubiquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient
- Gene Name:
- nuoL
- Locus Tag:
- PA2647
- Molecular weight:
- 66.2 kDa
Reactions
| NADH + quinone = NAD(+) + quinol. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Catalytic subunit of the periplasmic nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme protein napC, thus allowing electron flow between membrane and periplasm. Essential function for nitrate assimilation and may have a role in anaerobic metabolism
- Gene Name:
- napA
- Locus Tag:
- PA1174
- Molecular weight:
- 92.9 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in malate dehydrogenase (quinone) activity
- Specific function:
- (S)-malate + a quinone = oxaloacetate + reduced quinone
- Gene Name:
- mqo
- Locus Tag:
- PA3452
- Molecular weight:
- 57.2 kDa
Reactions
| (S)-malate + a quinone = oxaloacetate + reduced quinone. |
- General function:
- Involved in iron-sulfur cluster binding
- Specific function:
- Specific function unknown
- Gene Name:
- glcF
- Locus Tag:
- PA5353
- Molecular weight:
- 44.7 kDa
- General function:
- Involved in coenzyme binding
- Specific function:
- Specific function unknown
- Gene Name:
- mdaB
- Locus Tag:
- PA2580
- Molecular weight:
- 22 kDa
- General function:
- Involved in catalytic activity
- Specific function:
- Specific function unknown
- Gene Name:
- glcE
- Locus Tag:
- PA5354
- Molecular weight:
- 38.2 kDa
- General function:
- Involved in protein disulfide oxidoreductase activity
- Specific function:
- Required for disulfide bond formation in some periplasmic proteins such as phoA or ompA. Acts by transferring its disulfide bond to other proteins and is reduced in the process. DsbA is reoxidized by dsbB. It is required for pilus biogenesis
- Gene Name:
- dsbA
- Locus Tag:
- PA5489
- Molecular weight:
- 23.4 kDa
- General function:
- Involved in methyltransferase activity
- Specific function:
- Methyltransferase required for the conversion of dimethylmenaquinone (DMKH2) to menaquinone (MKH2) and the conversion of 2-polyprenyl-6-methoxy-1,4-benzoquinol (DDMQH2) to 2-polyprenyl-3-methyl-6-methoxy-1,4-benzoquinol (DMQH2)
- Gene Name:
- ubiE
- Locus Tag:
- PA5063
- Molecular weight:
- 28.3 kDa
Reactions
| A demethylmenaquinone + S-adenosyl-L-methionine = a menaquinol + S-adenosyl-L-homocysteine. |
| S-adenosyl-L-methionine + 2-methoxy-6-all-trans-polyprenyl-1,4-benzoquinol = S-adenosyl-L-homocysteine + 6-methoxy-3-methyl-2-all-trans-polyprenyl-1,4-benzoquinol. |
- General function:
- Energy production and conversion
- Specific function:
- Small subunit of the periplasmic nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme napC protein, thus allowing electron flow between membrane and periplasm. Essential function for nitrate assimilation and may have a role in anaerobic metabolism
- Gene Name:
- napB
- Locus Tag:
- PA1173
- Molecular weight:
- 17.9 kDa
- General function:
- Involved in protein disulfide oxidoreductase activity
- Specific function:
- Required for disulfide bond formation in some periplasmic proteins such as phoA or ompA. Acts by oxidizing the dsbA protein
- Gene Name:
- dsbB
- Locus Tag:
- PA0538
- Molecular weight:
- 18.1 kDa
- General function:
- Involved in heme binding
- Specific function:
- Mediates electron flow from quinones to the napAB complex
- Gene Name:
- napC
- Locus Tag:
- PA1172
- Molecular weight:
- 22.7 kDa
- General function:
- Involved in unfolded protein binding
- Specific function:
- Chaperone required for proper molybdenum cofactor insertion and final assembly of the membrane-bound respiratory nitrate reductase 1. Required for the insertion of the molybdenum into the apo-NarG subunit, maybe by keeping NarG in an appropriate competent-open conformation for the molybdenum cofactor insertion to occur. NarJ maintains the apoNarGH complex in a soluble state. Upon insertion of the molybdenum cofactor, NarJ seems to dissociate from the activated soluble NarGH complex, before its association with the NarI subunit on the membrane
- Gene Name:
- narJ
- Locus Tag:
- PA3873
- Molecular weight:
- 27.3 kDa