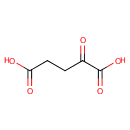
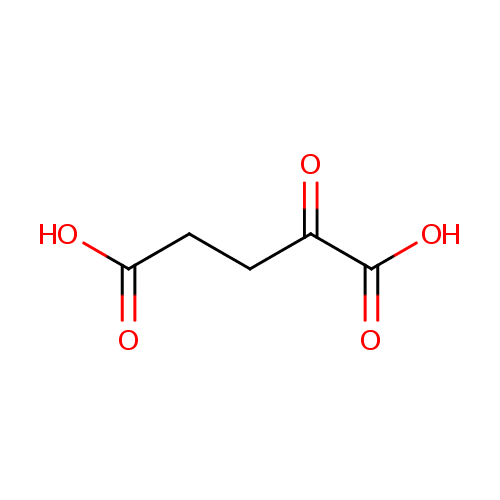
| References: |
- Bales JR, Higham DP, Howe I, Nicholson JK, Sadler PJ: Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clin Chem. 1984 Mar;30(3):426-32. Pubmed: 6321058
- Barbas C, Garcia A, de Miguel L, Simo C: Evaluation of filter paper collection of urine samples for detection and measurement of organic acidurias by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Nov 15;780(1):73-82. Pubmed: 12383482
- Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J., Rabinowitz, J. D. (2009). "Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli." Nat Chem Biol 5:593-599. Pubmed: 19561621
- Garcia A, Barbas C, Aguilar R, Castro M: Capillary electrophoresis for rapid profiling of organic acidurias. Clin Chem. 1998 Sep;44(9):1905-11. Pubmed: 9732975
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. Pubmed: 8087979
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Kelley RI: Octenylsuccinic aciduria in children fed protein-hydrolysate formulas containing modified cornstarch. Pediatr Res. 1991 Dec;30(6):564-9. Pubmed: 1805153
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kitaura J, Miki Y, Kato H, Sakakihara Y, Yanagisawa M: Hyperinsulinaemic hypoglycaemia associated with persistent hyperammonaemia. Eur J Pediatr. 1999 May;158(5):410-3. Pubmed: 10333126
- Lee SH, Kim SO, Chung BC: Gas chromatographic-mass spectrometric determination of urinary oxoacids using O-(2,3,4,5,6-pentafluorobenzyl)oxime-trimethylsilyl ester derivatization and cation-exchange chromatography. J Chromatogr B Biomed Sci Appl. 1998 Nov 20;719(1-2):1-7. Pubmed: 9869358
- Nicholson JK, Foxall PJ, Spraul M, Farrant RD, Lindon JC: 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal Chem. 1995 Mar 1;67(5):793-811. Pubmed: 7762816
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. Pubmed: 12829005
- Rocchiccioli F, Leroux JP, Cartier PH: Microdetermination of 2-ketoglutaric acid in plasma and cerebrospinal fluid by capillary gas chromatography mass spectrometry; application to pediatrics. Biomed Mass Spectrom. 1984 Jan;11(1):24-8. Pubmed: 6704500
- Stromme JH, Borud O, Moe PJ: Fatal lactic acidosis in a newborn attributable to a congenital defect of pyruvate dehydrogenase. Pediatr Res. 1976 Jan;10(1):62-6. Pubmed: 813176
- Sweatman BC, Farrant RD, Holmes E, Ghauri FY, Nicholson JK, Lindon JC: 600 MHz 1H-NMR spectroscopy of human cerebrospinal fluid: effects of sample manipulation and assignment of resonances. J Pharm Biomed Anal. 1993 Aug;11(8):651-64. Pubmed: 8257730
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
- Zupke C, Sinskey AJ, Stephanopoulos G: Intracellular flux analysis applied to the effect of dissolved oxygen on hybridomas. Appl Microbiol Biotechnol. 1995 Dec;44(1-2):27-36. Pubmed: 8579834
|
|---|