Search Results for compounds
Searching compounds for
returned 4373 results.
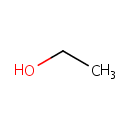
Ethanol (PAMDB000041)
IUPAC:
ethanol
CAS: 64-17-5
Description: Ethanol is a clear, colorless liquid. Many bacteria species, including gut microflora such as Pseudomonas aeruginosa, produce ethanol through fermentation in glucose metabolism. (HMDB, KEGG)
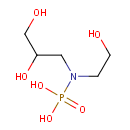
Glycerylphosphorylethanolamine (PAMDB000042)
IUPAC:
[(2,3-dihydroxypropyl)(2-hydroxyethyl)amino]phosphonic acid
CAS: 1190-00-7
Description: Glycerylphosphorylethanolamine is membrane breakdown product resulting from the cleavage of the lipid group from glycerophosphoethanlomine fatty acids (i.e. phosphatidylethanolamine). Phosphatidylethanolamine is one of the major lipid constituents of Pseudomonas aeruginosa.
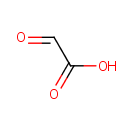
Glyoxylic acid (PAMDB000043)
IUPAC:
2-oxoacetic acid
CAS: 298-12-4
Description: Glyoxylic acid or oxoacetic acid is an organic compound that is both an aldehyde and a carboxylic acid. It is an intermediate of the glyoxylate cycle, which enables certain organisms to convert fatty acids into carbohydrates.The conjugate base of gloxylic acid is known as glyoxylate. This compound is an intermediate of the glyoxylate cycle, which enables organisms, such as bacteria, fungi and plants to convert fatty acids into carbohydrates. Glyoxylate is the byproduct of the amidation process in biosynthesis of several amidated peptides. The glyoxylate cycle is a metabolic pathway occurring in plants, and several microorganisms, such as Pseudomonas aeruginosa and yeast.
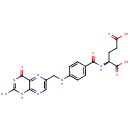
Folic acid (PAMDB000044)
IUPAC:
(2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid
CAS: 59-30-3
Description: Folic acid is a member of the vitamin B family. Folic acid, being biochemically inactive, is converted to tetrahydrofolic acid and methyltetrahydrofolate by dihydrofolate reductase. These folic acid congeners are transported across cells by receptor-mediated endocytosis, synthesize purine and thymidylate nucleic acids, interconvert amino acids, methylated tRNA, and generate and use formate.
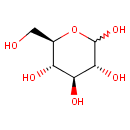
D-Glucose (PAMDB000045)
IUPAC:
(3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol
CAS: 50-99-7
Description: Glucose is a monosaccharide containing six carbon atoms and an aldehyde group and is therefore referred to as an aldohexose. The glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form, the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. In water solution both forms are in equilibrium and at pH 7 the cyclic one is the predominant. Glucose is a primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state.
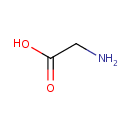
Glycine (PAMDB000046)
IUPAC:
2-aminoacetic acid
CAS: 56-40-6
Description: Glycine is a simple amino acid. The glycine cleavage enzyme system comprises four proteins: P-, T-, H- and L-proteins (EC 1.4.4.2, EC 2.1.2.10 and EC 1.8.1.4 for P-, T- and L-proteins). The glycine cleavage system catalyses the oxidative conversion of glycine into carbon dioxide and ammonia, with the remaining one-carbon unit transferred to folate as methylenetetrahydrofolate. It is the main catabolic pathway for glycine and it also contributes to one-carbon metabolism.
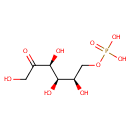
Fructose 6-phosphate (PAMDB000047)
IUPAC:
{[(2R,3R,4S)-2,3,4,6-tetrahydroxy-5-oxohexyl]oxy}phosphonic acid
CAS: 643-13-0
Description: Fructose-6-phosphate is an important intermediate in glycolysis and gluconeogenesis. The interconversion of glucose-6-phosphate and fructose-6-phosphate, the second step of the Embden-Meyerhof glycolytic pathway, is catalyzed by the enzyme phosphoglucose isomerase (PGI). In gluconeogenesis, fructose-6-phosphate is the immediate precursor of glucose-6-phosphate (wikipedia)
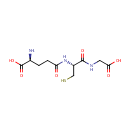
Glutathione (PAMDB000048)
IUPAC:
(2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic acid
CAS: 70-18-8
Description: Glutathione (GSH) is a compound synthesized from cysteine. Like cysteine, glutathione contains the crucial thiol (-SH) group that makes it an effective antioxidant. There are virtually no living organisms on this planet-animal or plant whose cells don't contain some glutathione. Scientists have speculated that glutathione was essential to the very development of life on earth. Glutathione has many roles; in none does it act alone. It is a coenzyme in various enzymatic reactions. The most important of these are redox reactions, in which the thiol grouping on the cysteine portion of cell membranes protects against peroxidation; and conjugation reactions, in which glutathione binds with toxic chemicals in order to detoxify them. GSH is known as a substrate in both conjugation reactions and reduction reactions, catalyzed by glutathione S-transferase enzymes in the bacterial cytosol.
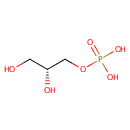
Glycerol 3-phosphate (PAMDB000049)
IUPAC:
[(2R)-2,3-dihydroxypropoxy]phosphonic acid
CAS: 57-03-4
Description: Glycerol 3-phosphate is a chemical intermediate in the glycolysis metabolic pathway. It is commonly confused with the similarly named glycerate 3-phosphate or glyceraldehyde 3-phosphate. Glycerol 3-phosphate is produced from glycerol, the triose sugar backbone of triglycerides and glycerophospholipids, by the enzyme glycerol kinase. Glycerol 3-phospate may then be converted by dehydrogenation to dihydroxyacetone phosphate (DHAP) by the enzyme glycerol-3-phosphate dehydrogenase. DHAP can then be rearranged into glyceraldehyde 3-phosphate (GA3P) by triose phosphate isomerase (TIM), and feed into glycolysis. (Wikipedia).
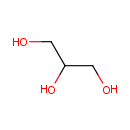
Glycerol (PAMDB000050)
IUPAC:
propane-1,2,3-triol
CAS: 56-81-5
Description: Glycerol is an organic compound, also called glycerin or glycerine. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydrophilic hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. Metabolism of glycerol in Pseudomonas aeruginosa, generally requires the presence of external electron acceptors. The respiratory pathways mediating this metabolic process involve a glycerol transporter (encoded by glpF), a glycerol kinase (encoded by glpK), and two respiratory glycerol-3-phosphate dehydrogenases (G3PDHs). Glycerol may also be metabolized fermentatively leading to the production of ethanol.