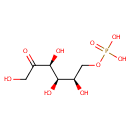
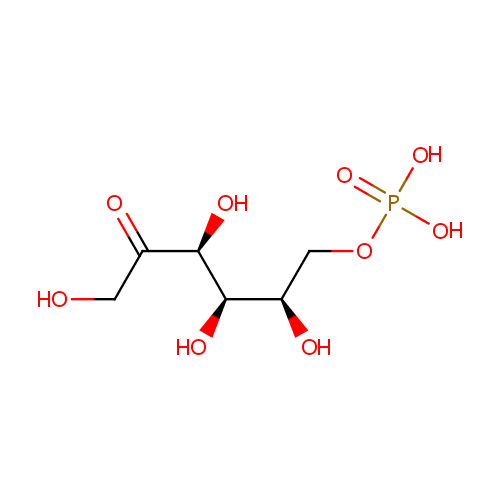
Fructose 6-phosphate (PAMDB000047)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000047 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Fructose 6-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Fructose-6-phosphate is an important intermediate in glycolysis and gluconeogenesis. The interconversion of glucose-6-phosphate and fructose-6-phosphate, the second step of the Embden-Meyerhof glycolytic pathway, is catalyzed by the enzyme phosphoglucose isomerase (PGI). In gluconeogenesis, fructose-6-phosphate is the immediate precursor of glucose-6-phosphate (wikipedia) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H13O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 260.1358 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 260.029718526 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | GSXOAOHZAIYLCY-HSUXUTPPSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H13O9P/c7-1-3(8)5(10)6(11)4(9)2-15-16(12,13)14/h4-7,9-11H,1-2H2,(H2,12,13,14)/t4-,5-,6-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 643-13-0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3R,4S)-2,3,4,6-tetrahydroxy-5-oxohexyl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | D-fructose 6-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OCC(=O)[C@@H](O)[C@H](O)[C@H](O)COP(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organophosphorus compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphoric acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phosphate esters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monoalkyl phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Fructose 6-phosphate <> Dihydroxyacetone + D-Glyceraldehyde 3-phosphate Phosphoenolpyruvic acid + D-Fructose > Fructose 6-phosphate + Pyruvic acid D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate <> D-Erythrose 4-phosphate + Fructose 6-phosphate Adenosine triphosphate + Fructose 6-phosphate > ADP + Fructose 1,6-bisphosphate + Hydrogen ion Fructose 1,6-bisphosphate + Water <> Fructose 6-phosphate + Phosphate Adenosine triphosphate + D-Fructose > ADP + Fructose 6-phosphate + Hydrogen ion Glucosamine 6-phosphate + Water > Fructose 6-phosphate + Ammonium Fructose 6-phosphate + Water > D-Fructose + Phosphate Mannose 6-phosphate <> Fructose 6-phosphate Sorbitol-6-phosphate + NAD <> Fructose 6-phosphate + Hydrogen ion + NADH Fructose 6-phosphate + L-Glutamine <> Glucosamine 6-phosphate + L-Glutamate Glucose 6-phosphate <> Fructose 6-phosphate D-Allulose-6-phosphate <> Fructose 6-phosphate Glucosamine 6-phosphate + Water <> Fructose 6-phosphate + Ammonia D-Erythrose 4-phosphate + Xylulose 5-phosphate <> Fructose 6-phosphate + D-Glyceraldehyde 3-phosphate Glucosamine 6-phosphate + Water <> Hydrogen ion + Fructose 6-phosphate + Ammonia D-fructose + Phosphoenolpyruvic acid > Fructose 6-phosphate + Pyruvic acid More...Fructose 1,6-bisphosphate + Water > Fructose 6-phosphate + Inorganic phosphate Adenosine triphosphate + Fructose 6-phosphate > ADP + Fructose 1,6-bisphosphate Adenosine triphosphate + D-Fructose > ADP + Fructose 6-phosphate Mannitol 1-phosphate + NAD > Fructose 6-phosphate + NADH Sorbitol-6-phosphate + NAD > Fructose 6-phosphate + NADH Sorbitol 6-phosphate + NAD <> Fructose 6-phosphate + NADH + Hydrogen ion Mannitol 1-phosphate + NAD <> Fructose 6-phosphate + NADH + Hydrogen ion beta-D-Glucose 6-phosphate > Fructose 6-phosphate + Fructose 6-phosphate Fructose 6-phosphate + Phosphate + Fructose 6-phosphate > Fructose 1,6-bisphosphate + Water + Fructose 1,6-bisphosphate Fructose 6-phosphate + Adenosine triphosphate + Fructose 6-phosphate > Fructose 1,6-bisphosphate + Adenosine diphosphate + Hydrogen ion + Fructose 1,6-bisphosphate + ADP Fructose 6-phosphate + L-Glutamine + Fructose 6-phosphate > L-Glutamic acid + Glucosamine 6-phosphate + L-Glutamate Fructose 6-phosphate + NADH + Hydrogen ion + Fructose 6-phosphate <> NAD + Mannitol 1-phosphate D-Fructose + HPr - phosphorylated + D-Fructose > HPr + Fructose 6-phosphate + Fructose 6-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidation-reduction process
- Specific function:
- D-mannitol 1-phosphate + NAD(+) = D-fructose 6-phosphate + NADH
- Gene Name:
- mtlD
- Locus Tag:
- PA2342
- Molecular weight:
- 54.3 kDa
Reactions
| D-mannitol 1-phosphate + NAD(+) = D-fructose 6-phosphate + NADH. |
- General function:
- Involved in glucose-6-phosphate isomerase activity
- Specific function:
- D-glucose 6-phosphate = D-fructose 6- phosphate
- Gene Name:
- pgi
- Locus Tag:
- PA4732
- Molecular weight:
- 61.9 kDa
Reactions
| D-glucose 6-phosphate = D-fructose 6-phosphate. |
- General function:
- Involved in catalytic activity
- Specific function:
- Transaldolase is important for the balance of metabolites in the pentose-phosphate pathway
- Gene Name:
- talB
- Locus Tag:
- PA2796
- Molecular weight:
- 33.9 kDa
Reactions
| Sedoheptulose 7-phosphate + D-glyceraldehyde 3-phosphate = D-erythrose 4-phosphate + D-fructose 6-phosphate. |
- General function:
- Involved in phosphoric ester hydrolase activity
- Specific function:
- D-fructose 1,6-bisphosphate + H(2)O = D- fructose 6-phosphate + phosphate
- Gene Name:
- fbp
- Locus Tag:
- PA5110
- Molecular weight:
- 37.2 kDa
Reactions
| D-fructose 1,6-bisphosphate + H(2)O = D-fructose 6-phosphate + phosphate. |
- General function:
- Involved in metabolic process
- Specific function:
- Catalyzes the first step in hexosamine metabolism, converting fructose-6P into glucosamine-6P using glutamine as a nitrogen source
- Gene Name:
- glmS
- Locus Tag:
- PA5549
- Molecular weight:
- 66.3 kDa
Reactions
| L-glutamine + D-fructose 6-phosphate = L-glutamate + D-glucosamine 6-phosphate. |
- General function:
- Involved in catalytic activity
- Specific function:
- Sedoheptulose 7-phosphate + D-glyceraldehyde 3-phosphate = D-ribose 5-phosphate + D-xylulose 5-phosphate
- Gene Name:
- tktA
- Locus Tag:
- PA0548
- Molecular weight:
- 72.2 kDa
Reactions
| Sedoheptulose 7-phosphate + D-glyceraldehyde 3-phosphate = D-ribose 5-phosphate + D-xylulose 5-phosphate. |
- General function:
- Not Available
- Specific function:
- Not Available
- Gene Name:
- cobB
- Locus Tag:
- PA1273
- Molecular weight:
- 46.5 kDa