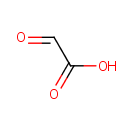
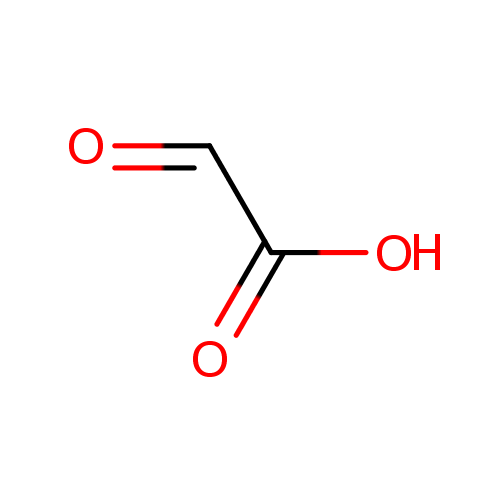
Glyoxylic acid (PAMDB000043)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000043 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Glyoxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Glyoxylic acid or oxoacetic acid is an organic compound that is both an aldehyde and a carboxylic acid. It is an intermediate of the glyoxylate cycle, which enables certain organisms to convert fatty acids into carbohydrates.The conjugate base of gloxylic acid is known as glyoxylate. This compound is an intermediate of the glyoxylate cycle, which enables organisms, such as bacteria, fungi and plants to convert fatty acids into carbohydrates. Glyoxylate is the byproduct of the amidation process in biosynthesis of several amidated peptides. The glyoxylate cycle is a metabolic pathway occurring in plants, and several microorganisms, such as Pseudomonas aeruginosa and yeast. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C2H2O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 74.0355 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 74.00039393 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | HHLFWLYXYJOTON-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C2H2O3/c3-1-2(4)5/h1H,(H,4,5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 298-12-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-oxoacetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | glyoxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(=O)C=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as carboxylic acids. These are compounds containing a carboxylic acid group with the formula -C(=O)OH. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carboxylic acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Carboxylic acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -93 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Glyoxylic acid + Hydrogen ion + NADPH + Glycolate <> Glycolic acid + NADP Glycolic acid + Ubiquinone-8 > Glyoxylic acid + Ubiquinol-8 Glycolic acid + Menaquinone 8 > Glyoxylic acid + Menaquinol 8 2-Demethylmenaquinone 8 + Glycolic acid > 2-Demethylmenaquinol 8 + Glyoxylic acid Glyoxylic acid + Hydrogen ion + NADH > Glycolic acid + NAD Acetyl-CoA + Glyoxylic acid + Water <> Coenzyme A + Hydrogen ion + L-Malic acid 2 Hydrogen ion + Water + (S)-Ureidoglycolic acid > Carbon dioxide + Glyoxylic acid +2 Ammonium 2 Glyoxylic acid + Hydrogen ion <> Tartronate semialdehyde + Carbon dioxide FMNH + Oxygen + Sulfoacetate > Flavin Mononucleotide + Glyoxylic acid + Hydrogen ion + Water + Sulfite Isocitric acid <> Glyoxylic acid + Succinic acid 2 Glyoxylic acid <> Tartronate semialdehyde + Carbon dioxide Glycolic acid + NADP <> Glyoxylic acid + NADPH + Hydrogen ion (S)-Ureidoglycolic acid + Water <> Glyoxylic acid +2 Ammonia + Carbon dioxide 4-Hydroxy-2-oxoglutaric acid <> Pyruvic acid + Glyoxylic acid D-4-Hydroxy-2-oxoglutarate <> Pyruvic acid + Glyoxylic acid L-Malic acid + Coenzyme A <> Acetyl-CoA + Water + Glyoxylic acid Glycolic acid + Oxygen <> Glyoxylic acid + Hydrogen peroxide Dehydroglycine + Water > Glyoxylic acid + Ammonia an oxidized electron acceptor + Glycolic acid > a reduced electron acceptor + Glyoxylic acid Propionyl-CoA + Water + Glyoxylic acid <> 2-hydroxyglutarate + Hydrogen ion + Coenzyme A (S)-Ureidoglycolic acid > Urea + Glyoxylic acid More...Glycolic acid + NADP > Glyoxylic acid + NADPH L-Alanine + Glyoxylic acid + L-Alanine <> Glycine + Pyruvic acid Glycolic acid + an oxidized electron-transfer flavoprotein? > Reduced acceptor + Glyoxylic acid 2 Glycolic acid + 2 an oxidized electron-transfer flavoprotein? >2 Reduced acceptor +2 Glyoxylic acid Glyoxylic acid + Water + Acetyl-CoA > Coenzyme A + Hydrogen ion + L-Malic acid + L-Malic acid Isocitric acid + Isocitric acid <> Succinic acid + Glyoxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Jie, Yuanping; Song, Zhen. Method for preparing glyoxylic acid. Faming Zhuanli Shenqing Gongkai Shuomingshu (2007), 5pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in isocitrate lyase activity
- Specific function:
- Catalyzes the formation of succinate and glyoxylate from isocitrate, a key step of the glyoxylate cycle. May be involved in the assimilation of one-carbon compounds via the isocitrate lyase- positive serine pathway
- Gene Name:
- aceA
- Locus Tag:
- PA2634
- Molecular weight:
- 58.9 kDa
Reactions
| Isocitrate = succinate + glyoxylate. |
- General function:
- Involved in tartronate-semialdehyde synthase activity
- Specific function:
- Catalyzes the condensation of two molecules of glyoxylate to give 2-hydroxy-3-oxopropanoate (also termed tartronate semialdehyde)
- Gene Name:
- gcl
- Locus Tag:
- PA1502
- Molecular weight:
- 64.4 kDa
Reactions
| 2 glyoxylate = tartronate semialdehyde + CO(2). |
- General function:
- Involved in catalytic activity
- Specific function:
- Specific function unknown
- Gene Name:
- glcD
- Locus Tag:
- PA5355
- Molecular weight:
- 53.7 kDa
- General function:
- Involved in catalytic activity
- Specific function:
- Accounts for almost the entire malate-synthesizing activity in cells metabolizing glyoxylate
- Gene Name:
- glcB
- Locus Tag:
- PA0482
- Molecular weight:
- 78.7 kDa
Reactions
| Acetyl-CoA + H(2)O + glyoxylate = (S)-malate + CoA. |
- General function:
- Involved in oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor
- Specific function:
- Catalyzes the NADPH-dependent reduction of glyoxylate and hydroxypyruvate into glycolate and glycerate, respectively. Can also reduce 2,5-diketo-D-gluconate (25DKG) to 5-keto-D- gluconate (5KDG), 2-keto-D-gluconate (2KDG) to D-gluconate, and 2- keto-L-gulonate (2KLG) to L-idonate (IA), but it is not its physiological function. Inactive towards 2-oxoglutarate, oxaloacetate, pyruvate, 5-keto-D-gluconate, D-fructose and L- sorbose. Activity with NAD is very low
- Gene Name:
- ghrB
- Locus Tag:
- PA2263
- Molecular weight:
- 35.6 kDa
Reactions
| Glycolate + NADP(+) = glyoxylate + NADPH. |
| D-glycerate + NAD(P)(+) = hydroxypyruvate + NAD(P)H. |
| D-gluconate + NADP(+) = 2-dehydro-D-gluconate + NADPH. |
- General function:
- Involved in iron-sulfur cluster binding
- Specific function:
- Specific function unknown
- Gene Name:
- glcF
- Locus Tag:
- PA5353
- Molecular weight:
- 44.7 kDa
- General function:
- Involved in ureidoglycolate hydrolase activity
- Specific function:
- Involved in the anaerobic utilization of allantoin. Reinforces the induction of genes involved in the degradation of allantoin and glyoxylate by producing glyoxylate
- Gene Name:
- allA
- Locus Tag:
- PA1514
- Molecular weight:
- 19 kDa
Reactions
| (S)-ureidoglycolate + H(2)O = glyoxylate + 2 NH(3) + CO(2). |
- General function:
- Involved in alkanesulfonate monooxygenase activity
- Specific function:
- Involved in desulfonation of aliphatic sulfonates. Catalyzes the conversion of pentanesulfonic acid to sulfite and pentaldehyde and is able to desulfonate a wide range of sulfonated substrates including C-2 to C-10 unsubstituted linear alkanesulfonates, substituted ethanesulfonic acids and sulfonated buffers
- Gene Name:
- ssuD
- Locus Tag:
- PA3444
- Molecular weight:
- 41.6 kDa
Reactions
| An alkanesufonate (R-CH(2)-SO(3)H) + FMNH(2) + O(2) = an aldehyde (R-CHO) + FMN + sulfite + H(2)O. |
- General function:
- Involved in catalytic activity
- Specific function:
- Specific function unknown
- Gene Name:
- glcE
- Locus Tag:
- PA5354
- Molecular weight:
- 38.2 kDa