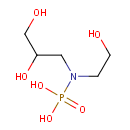
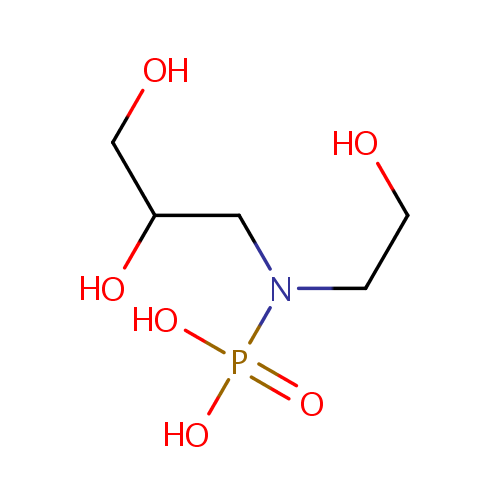
Glycerylphosphorylethanolamine (PAMDB000042)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000042 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Glycerylphosphorylethanolamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Glycerylphosphorylethanolamine is membrane breakdown product resulting from the cleavage of the lipid group from glycerophosphoethanlomine fatty acids (i.e. phosphatidylethanolamine). Phosphatidylethanolamine is one of the major lipid constituents of Pseudomonas aeruginosa. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H14NO6P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 215.1415 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 215.055873697 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | FRMZOWIQVCBEAC-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H14NO6P/c7-2-1-6(13(10,11)12)3-5(9)4-8/h5,7-9H,1-4H2,(H2,10,11,12) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 1190-00-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [(2,3-dihydroxypropyl)(2-hydroxyethyl)amino]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | GPEA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OCCN(CC(O)CO)P(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as organic phosphoric acids and derivatives. These are organic compounds containing phosphoric acid or a derivative therof. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organophosphorus compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphoric acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organic phosphoric acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Water + Glycerylphosphorylethanolamine > ADP + Glycerylphosphorylethanolamine + Hydrogen ion + Phosphate Adenosine triphosphate + Water + Glycerylphosphorylethanolamine > ADP + Glycerylphosphorylethanolamine + Hydrogen ion + Phosphate 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C12:0) + Water > Dodecanoate (N-C12:0) + Glycerylphosphorylethanolamine + Hydrogen ion 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:0) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + tetradecanoate (n-C14:0) 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:1) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Tetradecenoate (N-C14:1) 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C16:0) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Palmitic acid 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C18:0) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Octadecanoate (N-C18:0) 1-Acyl-sn-glycero-3-phosphoethanolamine (N-C18:1) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Octadecenoate (N-C18:1) Glycerylphosphorylethanolamine + Water > Ethanolamine + Glycerol 3-phosphate + Hydrogen ion 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C12:0) + Water > Dodecanoate (N-C12:0) + Glycerylphosphorylethanolamine + Hydrogen ion 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:0) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + tetradecanoate (n-C14:0) 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:1) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Tetradecenoate (N-C14:1) 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:1) + PG(14:1(7Z)/14:1(7Z)) > Acyl phosphatidylglycerol (N-C14:1) + Glycerylphosphorylethanolamine 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C16:0) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Palmitic acid 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C16:1) + PG(16:1(9Z)/16:1(9Z)) > Acyl phosphatidylglycerol (N-C16:1) + Glycerylphosphorylethanolamine 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C18:0) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Octadecanoate (N-C18:0) 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C18:1) + Water > Glycerylphosphorylethanolamine + Hydrogen ion + Octadecenoate (N-C18:1) 2-Acyl-sn-glycero-3-phosphoethanolamine (N-C18:1) + PG(18:1(11Z)/18:1(11Z)) > Acyl phosphatidylglycerol (N-C18:1) + Glycerylphosphorylethanolamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Tronconi, Giovanni. Process for the chromatographic isolation of 1-(a)-glycerylphosphorylcholine and of L-(a)-glycerylphosphorylethanolamine. PCT Int. Appl. (1990), 18 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Lipid transport and metabolism
- Specific function:
- 2-lysophosphatidylcholine + H(2)O = glycerophosphocholine + a carboxylate
- Gene Name:
- pldB
- Locus Tag:
- PA5089
- Molecular weight:
- 83.4 kDa
Reactions
| 2-lysophosphatidylcholine + H(2)O = glycerophosphocholine + a carboxylate. |
- General function:
- Involved in glycerophosphodiester phosphodiesterase activity
- Specific function:
- Glycerophosphoryl diester phosphodiesterase hydrolyzes deacylated phospholipids to G3P and the corresponding alcohols
- Gene Name:
- glpQ
- Locus Tag:
- PA0347
- Molecular weight:
- 42 kDa
Reactions
| A glycerophosphodiester + H(2)O = an alcohol + sn-glycerol 3-phosphate. |
- General function:
- Involved in hydrolase activity
- Specific function:
- Hydrolyzes only long chain acyl thioesters (C12-C18). Specificity similar to chymotrypsin
- Gene Name:
- tesA
- Locus Tag:
- PA2856
- Molecular weight:
- 21 kDa
Reactions
| 2-lysophosphatidylcholine + H(2)O = glycerophosphocholine + a carboxylate. |
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the ABC transporter complex UgpABCE involved in sn-glycerol-3-phosphate import. Responsible for energy coupling to the transport system (Probable). Can also transport glycerophosphoryl diesters
- Gene Name:
- ugpC
- Locus Tag:
- PA3187
- Molecular weight:
- 42.2 kDa
Reactions
| ATP + H(2)O + glycerol-3-phosphate(Out) = ADP + phosphate + glycerol-3-phosphate(In). |
Transporters
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the ABC transporter complex UgpABCE involved in sn-glycerol-3-phosphate import. Responsible for energy coupling to the transport system (Probable). Can also transport glycerophosphoryl diesters
- Gene Name:
- ugpC
- Locus Tag:
- PA3187
- Molecular weight:
- 42.2 kDa
Reactions
| ATP + H(2)O + glycerol-3-phosphate(Out) = ADP + phosphate + glycerol-3-phosphate(In). |