Search Results for compounds
Searching compounds for
returned 4373 results.
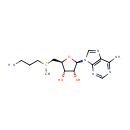
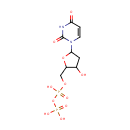
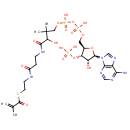
S-Adenosylmethioninamine (PAMDB000216)
IUPAC:
{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}(3-aminopropyl)methylsulfanium
CAS: 22365-13-5
Description: S-Adenosylmethioninamine is a biological sulfonium compound known as the major biological methyl donor. It is also a donor of methylene groups, amino groups, ribosyl groups and aminopropyl groups (PMID 15130560). S-Adenosylmethioninamine is a prodcut of enzyme adenosylmethionine decarboxylase [EC 4.1.1.50] in methionine metabolism pathway (KEGG).
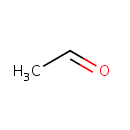
Acetaldehyde (PAMDB000217)
IUPAC:
acetaldehyde
CAS: 75-07-0
Description: Acetaldehyde is a colorless, flammable liquid used in the manufacture of acetic acid, perfumes, and flavors. It is also an intermediate in the metabolism of alcohol. Small amounts of acetaldehyde are produced naturally through gut microbial fermentation. Acetaldehyde is produced through the action of alcohol dehydrogenase on ethanol and is somewhate more toxic than ethanol.
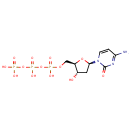
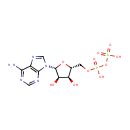
dCTP (PAMDB000219)
IUPAC:
({[({[(2R,3S,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid
CAS: 2056-98-6
Description: Deoxycytidine triphosphate (dCTP) is a cytidine nucleotide triphosphate that is used whenever DNA is synthesized, such as in the polymerase chain reaction. e.g.:
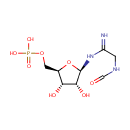
Phosphoribosylformylglycineamidine (PAMDB000220)
IUPAC:
{[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2-formamidoethanimidamido)oxolan-2-yl]methoxy}phosphonic acid
CAS: 37721-04-3
Description: 5-Phosphoribosyl-n-formylglycineamidine is part of the 5-aminoimidazole ribonucleotide biosynthesis pathway. 5-amino-1-(5-phospho-D-ribosyl)imidazole (AIR) is a key intermediate in the biosynthesis of purine nocleotides and thiamine. It is synthesized from 5-phospho-alpha-D-ribose 1-diphosphate (PRPP) in 5 steps, catalyzed by the enzymes amidophosphoribosyl transferase, phosphoribosylamine-glycine ligase, phosphoribosylglycinamide formyltransferase, phosphoribosylformylglycinamide synthetase and phosphoribosylformylglycinamide cyclo-ligase.
dUDP (PAMDB000221)
IUPAC:
[({[5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid
CAS: Not Available
Description: dUDP is a member of the chemical class known as Pyrimidine 2'-deoxyribonucleoside Diphosphates. These are pyrimidine nucleotides with a diphosphate group linked to the ribose moiety lacking an hydroxyl group at position 2. Deoxyuridine Diphosphate (dUDP) dUDP is a deoxyribonucleotide made from UDP in the reaction catalyzed by ribonucleotide reductase, as follows: UDP + NADPH <=> dUDP + NADP+ (http://www.pearsonhighered.com/mathews/molex/dudp.htm)
Adenosine phosphosulfate (PAMDB000222)
IUPAC:
[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]sulfonic acid
CAS: 485-84-7
Description: Adenosine phosphosulfate (also known as APS) is the initial compound formed by the action of ATP sulfurylase (or PAPS synthetase) on sulfate ions after sulfate uptake. PAPS synthetase 1 is a bifunctional enzyme with both ATP sulfurylase and APS kinase activity, which mediates two steps in the sulfate activation pathway. The first step is the transfer of a sulfate group to ATP to yield adenosine 5'-phosphosulfate (APS), and the second step is the transfer of a phosphate group from ATP to APS yielding 3'-phosphoadenylylsulfate (PAPS).
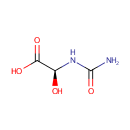
(S)-Ureidoglycolic acid (PAMDB000223)
IUPAC:
(2S)-2-(carbamoylamino)-2-hydroxyacetic acid
CAS: 7424-03-5
Description: (S)-Ureidoglycolic acid is a substrate of enzyme ureidoglycolate dehydrogenase [EC 1.1.1.154] in purine metabolism pathway (KEGG). Enteric bacteria such as Pseudomonas aeruginosa are able to utilize allantoin as a sole source of nitrogen under anaerobic conditions, but cannot utilize it as a sole source of carbon The first step in allantoin degradation is the opening of the aromatic ring, yielding allantoate, performed by allantoinase. In the next step allantoate is hydrolyzed to S-ureidoglycine by allantoate amidohydrolase. Ureidoglycine spontaneously converts to Ureidoglycolate. Ureidoglycolate dehydrogenase then oxidizes ureidoglycolate to oxalurate. It is believed oxalurate is converted into oxamate and carbamoyl-phosphate, which can be further metabolized to CO2, ammonia and ATP.
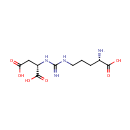
Argininosuccinic acid (PAMDB000224)
IUPAC:
(2S)-2-{3-[(4S)-4-amino-4-carboxybutyl]carbamimidamido}butanedioic acid
CAS: 2387-71-5
Description: Arginosuccinic acid is a basic amino acid. Some cells synthesize it from citrulline, aspartic acid and use it as a precursor for arginine in the urea cycle or citrulline-NO cycle. The enzyme that catalyzes the reaction is argininosuccinate synthetase. Argininosuccinic acid is a precursor to fumarate in the citric acid cycle via argininosuccinate lyase.
Methacrylyl-CoA (PAMDB000225)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy(3-hydroxy-2,2-dimethyl-3-{[2-({2-[(2-methylprop-2-enoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy)phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 6008-91-9
Description: Methacrylyl-CoA is a metabolite in the valine, leucine and isoleucine degradation pathway and is highly reactive with free thiol compounds (PMID 14684172; KEGG).
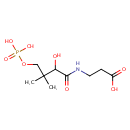
D-4'-Phosphopantothenate (PAMDB000227)
IUPAC:
3-{2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido}propanoic acid
CAS: Not Available
Description: D-4'-Phosphopantothenate is a product of the phosphorylatation of Pantothenate by Pantothenate kinase. It is an intermediate in coenzyme A (CoA) biosynthesis pathway. Coenzyme A is a cofactor of ubiquitous occurrence in plants, bacteria, and animals. It is needed in a large number of enzymatic reactions central to intermediary metabolism, including the oxidation of fatty acids, carbohydrates, and amino acids. Only plants and microorganisms like Pseudomonas aeruginosa are capable of synthesizing this compound de novo.