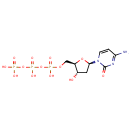
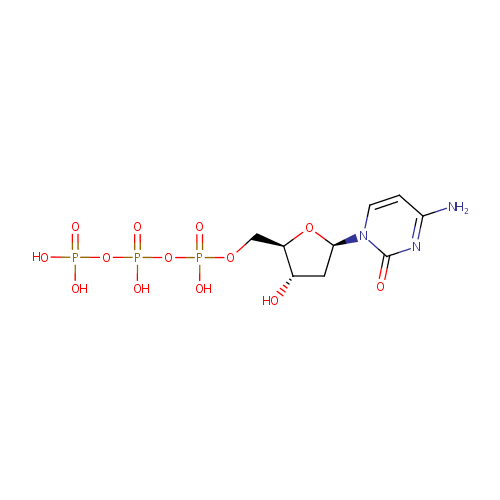
dCTP (PAMDB000219)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000219 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | dCTP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Deoxycytidine triphosphate (dCTP) is a cytidine nucleotide triphosphate that is used whenever DNA is synthesized, such as in the polymerase chain reaction. e.g.: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H16N3O13P3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 467.1569 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 466.989597149 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | RGWHQCVHVJXOKC-SHYZEUOFSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H16N3O13P3/c10-7-1-2-12(9(14)11-7)8-3-5(13)6(23-8)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8,13H,3-4H2,(H,18,19)(H,20,21)(H2,10,11,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2056-98-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | ({[({[(2R,3S,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | dCTP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC(=O)N(C=C1)[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleosides. These are compounds consisting of a pyrimidine linked to a ribose which lacks a hydroxyl group at position 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine 2'-deoxyribonucleosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine 2'-deoxyribonucleosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Cytidine triphosphate + 2 Flavodoxin reduced + 2 Hydrogen ion > dCTP +2 flavodoxin semi oxidized + Water Adenosine triphosphate + dCDP <> ADP + dCTP dCTP + Water > dCMP + Hydrogen ion + Pyrophosphate dCTP + Hydrogen ion + Water > Deoxyuridine triphosphate + Ammonium dCTP + DNA <> Pyrophosphate + DNA dCTP + Thioredoxin disulfide + Water <> Cytidine triphosphate + Thioredoxin dCTP + Uridine <> dCDP + Uridine 5'-monophosphate dCTP + Cytidine <> dCDP + Cytidine monophosphate Water + dCTP > Ammonia + Deoxyuridine triphosphate Cytidine triphosphate + Water + dCTP <> Cytidine monophosphate + Pyrophosphate + dCMP dCDP + Adenosine triphosphate > Adenosine diphosphate + dCTP + ADP Cytidine triphosphate + a reduced flavodoxin > Water + an oxidized flavodoxin + dCTP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Hinz M; Gottschling D; Eritja R; Seliger H Synthesis and properties of 2'-deoxycytidine triphosphate carrying c-myc tag sequence. Nucleosides, nucleotides & nucleic acids (2000), 19(10-12), 1543-52. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in nucleic acid binding
- Specific function:
- In addition to polymerase activity, this DNA polymerase exhibits 3' to 5' and 5' to 3' exonuclease activity. It is able to utilize nicked circular duplex DNA as a template and can unwind the parental DNA strand from its template
- Gene Name:
- polA
- Locus Tag:
- PA5493
- Molecular weight:
- 99.8 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in DNA binding
- Specific function:
- DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. The epsilon subunit contain the editing function and is a proofreading 3'-5' exonuclease
- Gene Name:
- dnaQ
- Locus Tag:
- PA1816
- Molecular weight:
- 26.8 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in nucleotide binding
- Specific function:
- The gamma chain seems to interact with the delta subunit to transfer the beta subunit on the DNA
- Gene Name:
- dnaX
- Locus Tag:
- PA1532
- Molecular weight:
- 73.3 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in nucleoside diphosphate kinase activity
- Specific function:
- Major role in the synthesis of nucleoside triphosphates other than ATP. The ATP gamma phosphate is transferred to the NDP beta phosphate via a ping-pong mechanism, using a phosphorylated active-site intermediate
- Gene Name:
- ndk
- Locus Tag:
- PA3807
- Molecular weight:
- 15.6 kDa
Reactions
| ATP + nucleoside diphosphate = ADP + nucleoside triphosphate. |
- General function:
- Involved in DNA binding
- Specific function:
- DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. This DNA polymerase also exhibits 3' to 5' exonuclease activity. The beta chain is required for initiation of replication once it is clamped onto DNA, it slides freely (bidirectional and ATP- independent) along duplex DNA
- Gene Name:
- dnaN
- Locus Tag:
- PA0002
- Molecular weight:
- 40.7 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in [formate-C-acetyltransferase]-activating enzyme activity
- Specific function:
- Activation of anaerobic ribonucleoside-triphosphate reductase under anaerobic conditions by generation of an organic free radical, using S-adenosylmethionine and reduced flavodoxin as cosubstrates to produce 5'-deoxy-adenosine
- Gene Name:
- nrdG
- Locus Tag:
- PA1919
- Molecular weight:
- 25.7 kDa
- General function:
- Involved in nucleoside-triphosphate diphosphatase activity
- Specific function:
- Specific function unknown
- Gene Name:
- mazG
- Locus Tag:
- PA0935
- Molecular weight:
- 31.2 kDa
Reactions
| ATP + H(2)O = AMP + diphosphate. |
- General function:
- Involved in 3'-5' exonuclease activity
- Specific function:
- DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. This DNA polymerase also exhibits 3' to 5' exonuclease activity. The alpha chain is the DNA polymerase
- Gene Name:
- dnaE
- Locus Tag:
- PA3640
- Molecular weight:
- 130.9 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in DNA binding
- Specific function:
- DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. This DNA polymerase also exhibits 3' to 5' exonuclease activity. The delta subunit seems to interact with the gamma subunit to transfer the beta subunit on the DNA
- Gene Name:
- holA
- Locus Tag:
- PA3989
- Molecular weight:
- 37.4 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in DNA binding
- Specific function:
- DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. This DNA polymerase also exhibits 3' to 5' exonuclease activity
- Gene Name:
- holB
- Locus Tag:
- PA2961
- Molecular weight:
- 35.7 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in catalytic activity
- Specific function:
- 2'-deoxyribonucleoside triphosphate + thioredoxin disulfide + H(2)O = ribonucleoside triphosphate + thioredoxin
- Gene Name:
- nrdD
- Locus Tag:
- PA1920
- Molecular weight:
- 76.1 kDa
Reactions
| 2'-deoxyribonucleoside triphosphate + thioredoxin disulfide + H(2)O = ribonucleoside triphosphate + thioredoxin. |
- General function:
- Involved in DNA binding
- Specific function:
- DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria. This DNA polymerase also exhibits 3' to 5' exonuclease activity
- Gene Name:
- holC
- Locus Tag:
- PA3832
- Molecular weight:
- 16.1 kDa
Reactions
| Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1). |
- General function:
- Involved in ATP binding
- Specific function:
- Catalyzes the reversible transfer of the terminal phosphate group between ATP and AMP. This small ubiquitous enzyme involved in the energy metabolism and nucleotide synthesis, is essential for maintenance and cell growth
- Gene Name:
- adk
- Locus Tag:
- PA3686
- Molecular weight:
- 23.1 kDa
Reactions
| ATP + AMP = 2 ADP. |