S-Adenosylmethioninamine (PAMDB000216)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000216 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | S-Adenosylmethioninamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | S-Adenosylmethioninamine is a biological sulfonium compound known as the major biological methyl donor. It is also a donor of methylene groups, amino groups, ribosyl groups and aminopropyl groups (PMID 15130560). S-Adenosylmethioninamine is a prodcut of enzyme adenosylmethionine decarboxylase [EC 4.1.1.50] in methionine metabolism pathway (KEGG). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
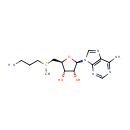
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C14H23N6O3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 355.436 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 355.155234322 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ZUNBITIXDCPNSD-LSRJEVITSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C14H23N6O3S/c1-24(4-2-3-15)5-8-10(21)11(22)14(23-8)20-7-19-9-12(16)17-6-18-13(9)20/h6-8,10-11,14,21-22H,2-5,15H2,1H3,(H2,16,17,18)/q+1/t8-,10-,11-,14-,24?/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 22365-13-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}(3-aminopropyl)methylsulfanium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | decarboxylated sam | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C[S+](CCCN)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as glycosylamines. These are compounds consisting of an?amine?with a?beta-N-glycosidic bond?to a carbohydrate, thus forming a cyclic?hemiaminal ether?bond (alpha-amino ether). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Glycosyl compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Glycosylamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | S-Adenosylmethionine + Hydrogen ion <> S-Adenosylmethioninamine + Carbon dioxide S-Adenosylmethioninamine + Putrescine + Ethylenediamine <> 5'-Methylthioadenosine + Hydrogen ion + Spermidine Cadaverine + S-Adenosylmethioninamine > 5'-Methylthioadenosine + Hydrogen ion + Aminopropylcadaverine S-Adenosylmethioninamine + Putrescine <> 5'-Methylthioadenosine + Spermidine S-Adenosylmethioninamine + Spermidine <> 5'-Methylthioadenosine + Spermine S-Adenosylmethioninamine + Cadaverine <> 5'-Methylthioadenosine + Aminopropylcadaverine Putrescine + S-Adenosylmethioninamine > Hydrogen ion + Spermidine + 5'-Methylthioadenosine S-adenosyl-L-methionine + Hydrogen ion > Carbon dioxide + S-Adenosylmethioninamine Putrescine + S-Adenosylmethioninamine > Spermidine + Hydrogen ion + 5'-S-methyl-5'-thioadenosine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Pegg, Anthony E. Assay of aminopropyltransferases. Methods in Enzymology (1983), 94(Polyamines), 260-5. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||