|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000221 |
|---|
|
Identification |
|---|
| Name: |
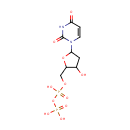
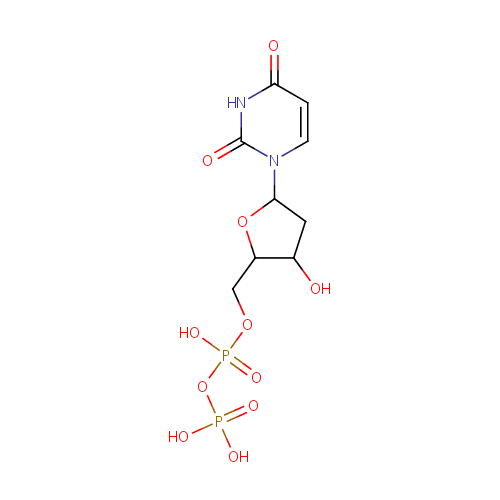
dUDP |
|---|
| Description: | dUDP is a member of the chemical class known as Pyrimidine 2'-deoxyribonucleoside Diphosphates. These are pyrimidine nucleotides with a diphosphate group linked to the ribose moiety lacking an hydroxyl group at position 2. Deoxyuridine Diphosphate (dUDP) dUDP is a deoxyribonucleotide made from UDP in the reaction catalyzed by ribonucleotide reductase, as follows: UDP + NADPH <=> dUDP + NADP+ (http://www.pearsonhighered.com/mathews/molex/dudp.htm) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2'-Deoxyuridine-5'-diphosphate
- 2'-Deoxyuridine-5'-diphosphoric acid
- Deoxyuridine-diphosphate
- Deoxyuridine-diphosphoric acid
- DUDP
|
|---|
|
Chemical Formula: |
C9H14N2O11P2 |
|---|
| Average Molecular Weight: |
388.1618 |
|---|
| Monoisotopic Molecular
Weight: |
388.007282324 |
|---|
| InChI Key: |
QHWZTVCCBMIIKE-SHYZEUOFSA-N |
|---|
| InChI: | InChI=1S/C9H14N2O11P2/c12-5-3-8(11-2-1-7(13)10-9(11)14)21-6(5)4-20-24(18,19)22-23(15,16)17/h1-2,5-6,8,12H,3-4H2,(H,18,19)(H,10,13,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [({[5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
|
Traditional IUPAC Name: |
deoxyuridine-5'-diphosphate |
|---|
| SMILES: | O[C@H]1C[C@@H](O[C@@H]1COP(O)(=O)OP(O)(O)=O)N1C=CC(=O)NC1=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleoside diphosphates. These are pyrimidine nucleotides with a diphosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine deoxyribonucleotides |
|---|
|
Direct Parent |
Pyrimidine 2'-deoxyribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine 2'-deoxyribonucleoside diphosphate
- Organic pyrophosphate
- Hydroxypyrimidine
- Monoalkyl phosphate
- Pyrimidone
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Hydropyrimidine
- Saccharide
- Heteroaromatic compound
- Oxolane
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB01000 | | Pubchem Compound ID | 687 | | Kegg ID | C01346 | | ChemSpider ID | 667 | | Wikipedia ID | Not Available | | BioCyc ID | DUDP | | EcoCyc ID | DUDP |
|
|---|