Search Results for compounds
Searching compounds for
returned 4373 results.
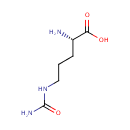
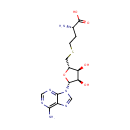
Citrulline (PAMDB000198)
IUPAC:
(2S)-2-amino-5-(carbamoylamino)pentanoic acid
CAS: 372-75-8
Description: Citrulline is an amino acid made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a by-product of the reaction catalyzed by NOS family (NOS; EC 1.14.13.39). In this reaction arginine is first oxidized into N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant with the release of nitric oxide.; EC 1.14.13.39). Citrulline's name is derived from citrullus, the Latin word for watermelon, from which it was first isolated.
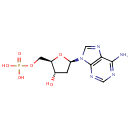
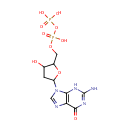
Deoxyadenosine monophosphate (PAMDB000199)
IUPAC:
{[(2R,3S,5R)-5-(6-amino-9H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy}phosphonic acid
CAS: 653-63-4
Description: Adenosine is a nucleoside comprised of adenine attached to a ribose (ribofuranose) moiety via a -N9-glycosidic bond.
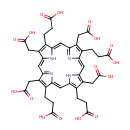
Uroporphyrin III (PAMDB000201)
IUPAC:
3-[10,14,19-tris(2-carboxyethyl)-5,9,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8(23),9,11,13,15,17,19-undecaen-4-yl]propanoic acid
CAS: 18273-06-8
Description: Uroporphyrin is the porphyrin produced by oxidation of the methylene bridges in uroporphyrinogen. Uroporphyrins have four acetic acid and four propionic acid side chains attached to the pyrrole rings. Uroporphyrinogen I and III are formed from polypyrryl methane in the presence of uroporphyrinogen III cosynthetase and uroporphyrin I synthetase, respectively. They can yield uroporphyrins by autooxidation or coproporphyrinogens by decarboxylation.
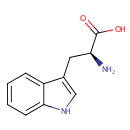
L-Tryptophan (PAMDB000203)
IUPAC:
(2S)-2-amino-3-(1H-indol-3-yl)propanoic acid
CAS: 73-22-3
Description: Tryptophan is an amino acid which is the precursor of serotonin. Plants and microorganisms commonly synthesize tryptophan from shikimic acid or anthranilate. The latter condenses with phosphoribosylpyrophosphate (PRPP), generating pyrophosphate as a by-product. After ring opening of the ribose moiety and following reductive decarboxylation, indole-3-glycerinephosphate is produced, which in turn is transformed into indole. In the last step, tryptophan synthase catalyzes the formation of tryptophan from indole and the amino acid, serine. Metabolism of tryptophan to serotonin requires nutrients such as vitamin B6, niacin and glutathione. Niacin is an important metabolite of tryptophan. (Wikipedia)
S-Adenosylhomocysteine (PAMDB000205)
IUPAC:
(2S)-2-amino-4-({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}sulfanyl)butanoic acid
CAS: 979-92-0
Description: S-Adenosylhomocysteine (AdoHcy) is the immediate precursor of homocysteine. The reaction is catalyzed by S-adenosylhomocysteine hydrolase and is reversible with the equilibrium favoring formation of AdoHcy. In vivo, the reaction is driven in the direction of homocysteine formation by the action of the enzyme adenosine deaminase, which converts the second product of the S-adenosylhomocysteine hydrolase reaction, adenosine, to inosine. Except for methyl transfer from betaine and from methylcobalamin in the methionine synthase reaction, AdoHcy is the product of all methylation reactions that involve S-adenosylmethionine (AdoMet) as the methyl donor.
dGDP (PAMDB000209)
IUPAC:
[({[5-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid
CAS: 102783-74-4
Description: Deoxyguanosine diphosphate or dGDP is a member of the chemical class known as Purine 2'-deoxyribonucleoside Diphosphates. These are purine nucleotides with diphosphate group linked to the ribose moiety lacking an hydroxyl group at position 2. DGDP is invovled in Purine metabolism. It is related to the common nucleic acid GTP, or guanosine triphosphate, with the -OH (hydroxyl) group on the 2' carbon on the nucleotide's pentose removed (hence the deoxy- part of the name), and with one fewer phosphoryl group than GTP.
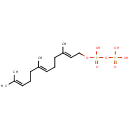
Farnesyl pyrophosphate (PAMDB000210)
IUPAC:
{[hydroxy({[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl]oxy})phosphoryl]oxy}phosphonic acid
CAS: 13058-04-3
Description: Farnesyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. -- Wikipedia
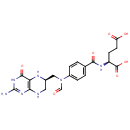
N10-Formyl-THF (PAMDB000213)
IUPAC:
(2S)-2-{[4-(N-{[(6R)-2-amino-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl]methyl}formamido)phenyl]formamido}pentanedioic acid
CAS: 2800-34-2
Description: N10-Formyl-THF is a substrate for Trifunctional purine biosynthetic protein adenosine-3, Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, 10-formyltetrahydrofolate dehydrogenase, Folylpolyglutamate synthase, Bifunctional purine biosynthesis protein PURH and C-1-tetrahydrofolate synthase (cytoplasmic).
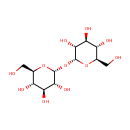
Trehalose (PAMDB000214)
IUPAC:
(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-3,4,5-triol
CAS: 99-20-7
Description: Trehalose, also known as mycose, is a 1-alpha (disaccharide) sugar found extensively but not abundantly in nature. It is thought to be implicated in anhydrobiosis - the ability of plants and animals to withstand prolonged periods of desiccation. The sugar is thought to form a gel phase as cells dehydrate, which prevents disruption of internal cell organelles by effectively splinting them in position. Rehydration then allows normal cellular activity to be resumed without the major, generally lethal damage that would normally follow a dehydration/reyhdration cycle.Trehalose is a non-reducing sugar formed from two glucose units joined by a 1-1 alpha bond giving it the name of alpha-D-glucopyranoglucopyranosyl-1,1-alpha-D-glucopyranoside. The bonding makes trehalose very resistant to acid hydrolysis, and therefore stable in solution at high temperatures even under acidic conditions. The bonding also keeps non-reducing sugars in closed-ring form, such that the aldehyde or ketone end-groups do not bind to the lysine or arginine residues of proteins (a process called glycation). The enzyme trehalase breaks it into two glucose molecules.
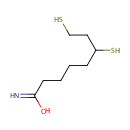
Dihydrolipoamide (PAMDB000215)
IUPAC:
6,8-disulfanyloctanimidic acid
CAS: 3884-47-7
Description: Dihydrolipoamide is a member of the chemical class known as N-acyl Amines. These are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. Dihydrolipoamide is a molecule produced by the action of dihydrolipoyl dehydrogenase upon lipoamide.