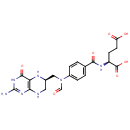
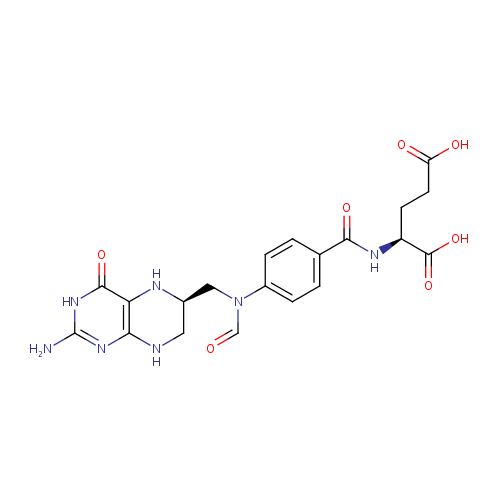
N10-Formyl-THF (PAMDB000213)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000213 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | N10-Formyl-THF | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | N10-Formyl-THF is a substrate for Trifunctional purine biosynthetic protein adenosine-3, Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, 10-formyltetrahydrofolate dehydrogenase, Folylpolyglutamate synthase, Bifunctional purine biosynthesis protein PURH and C-1-tetrahydrofolate synthase (cytoplasmic). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C20H23N7O7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 473.4393 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 473.165896125 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | AUFGTPPARQZWDO-YUZLPWPTSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C20H23N7O7/c21-20-25-16-15(18(32)26-20)23-11(7-22-16)8-27(9-28)12-3-1-10(2-4-12)17(31)24-13(19(33)34)5-6-14(29)30/h1-4,9,11,13,23H,5-8H2,(H,24,31)(H,29,30)(H,33,34)(H4,21,22,25,26,32)/t11?,13-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2800-34-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-{[4-(N-{[(6R)-2-amino-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl]methyl}formamido)phenyl]formamido}pentanedioic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 10-formyltetrahydrofolic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC(=O)C2=C(NCC(CN(C=O)C3=CC=C(C=C3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)N2)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as hippuric acids. These are compounds containing hippuric acid, which consists of a of a benzoyl group linked to the N-terminal of a glycine. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Benzenoids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzene and substituted derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Benzamides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Hippuric acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Water + 5,10-Methenyltetrahydrofolate <> N10-Formyl-THF + Hydrogen ion N10-Formyl-THF + Water <> Formic acid + Hydrogen ion + Tetrahydrofolic acid N10-Formyl-THF + Uridine 5''-diphospho-{beta}-4-deoxy-4-amino-L-arabinose <> Hydrogen ion + Tetrahydrofolic acid + Uridine 5''-diphospho-{beta}-4-deoxy-4-formamido-L-arabinose N10-Formyl-THF + Glycineamideribotide <> 5'-Phosphoribosyl-N-formylglycineamide + Hydrogen ion + Tetrahydrofolic acid N10-Formyl-THF + L-Methionyl-tRNA (Met) > N-Formylmethionyl-tRNA + Hydrogen ion + Tetrahydrofolic acid N10-Formyl-THF + Phosphoribosyl formamidocarboxamide <> Phosphoribosyl formamidocarboxamide + Tetrahydrofolic acid N10-Formyl-THF + Water <> Formic acid + Tetrahydrofolic acid L-Methionyl-tRNA + N10-Formyl-THF <> Tetrahydrofolic acid + N-Formylmethionyl-tRNA N10-Formyl-THF + Glycineamideribotide <> Tetrahydrofolic acid + 5'-Phosphoribosyl-N-formylglycineamide N10-Formyl-THF + AICAR <> Tetrahydrofolic acid + Phosphoribosyl formamidocarboxamide N10-Formyl-THF + Uridine 5''-diphospho-{beta}-4-deoxy-4-amino-L-arabinose <> Tetrahydrofolic acid + Uridine 5''-diphospho-{beta}-4-deoxy-4-formamido-L-arabinose Adenosine triphosphate + Formic acid + Tetrahydrofolic acid > ADP + Phosphate + N10-Formyl-THF N10-Formyl-THF + 4-Amino-4-deoxy-L-arabinose > Tetrahydrofolic acid + UDP-4-Deoxy-4-formamido-beta-L-arabinose N10-Formyl-THF + L-methionyl-tRNA(fMet) > Tetrahydrofolic acid + N-formylmethionyl-tRNA(fMet) 5,10-Methenyltetrahydrofolate + Water > N10-Formyl-THF Tetrahydrofolic acid + FAICAR + Tetrahydrofolic acid > 10-Formyltetrahydrofolate + AICAR + N10-Formyl-THF Tetrahydrofolic acid + 5'-Phosphoribosyl-N-formylglycinamide + Tetrahydrofolic acid + 5'-Phosphoribosyl-N-formylglycineamide > 10-Formyltetrahydrofolate + Glycineamideribotide + N10-Formyl-THF + Glycineamideribotide More...Formic acid + Tetrahydrofolic acid + Tetrahydrofolic acid > Water + 10-Formyltetrahydrofolate + N10-Formyl-THF Tetrahydrofolic acid + N-formylmethionyl-tRNA(fMet) + Tetrahydrofolic acid L-methionyl-tRNA(Met) + 10-Formyltetrahydrofolate + N10-Formyl-THF Uridine 5''-diphospho-{beta}-4-deoxy-4-amino-L-arabinose + an N10-formyl-tetrahydrofolate + N10-Formyl-THF > UDP-4-Deoxy-4-formamido-beta-L-arabinose + Hydrogen ion + a tetrahydrofolate + Tetrahydrofolic acid AICAR + 10-Formyltetrahydrofolate + N10-Formyl-THF > FAICAR + tetrahydropteroyl mono-L-glutamate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Denis V; Daignan-Fornier B Synthesis of glutamine, glycine and 10-formyl tetrahydrofolate is coregulated with purine biosynthesis in Saccharomyces cerevisiae. Molecular & general genetics : MGG (1998), 259(3), 246-55. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||