|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000209 |
|---|
|
Identification |
|---|
| Name: |
dGDP |
|---|
| Description: | Deoxyguanosine diphosphate or dGDP is a member of the chemical class known as Purine 2'-deoxyribonucleoside Diphosphates. These are purine nucleotides with diphosphate group linked to the ribose moiety lacking an hydroxyl group at position 2. DGDP is invovled in Purine metabolism. It is related to the common nucleic acid GTP, or guanosine triphosphate, with the -OH (hydroxyl) group on the 2' carbon on the nucleotide's pentose removed (hence the deoxy- part of the name), and with one fewer phosphoryl group than GTP. |
|---|
|
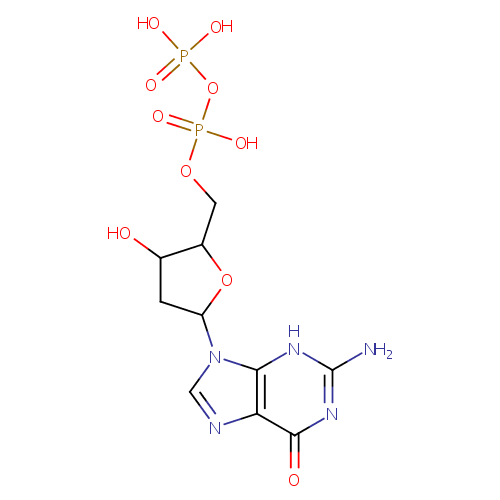
Structure |
|
|---|
| Synonyms: | - 2'-Deoxy-GDP
- 2'-Deoxyguanosine-5'-diphosphate
- 2'-Deoxyguanosine-5'-diphosphoric acid
- 5'-dGDP
- Deoxyguanosine 5'-diphosphate
- Deoxyguanosine 5'-diphosphoric acid
- Deoxyguanosine diphosphate
- Deoxyguanosine diphosphoric acid
- Deoxyguanosine-diphosphate
- Deoxyguanosine-diphosphoric acid
- DGDP
|
|---|
|
Chemical Formula: |
C10H15N5O10P2 |
|---|
| Average Molecular Weight: |
427.2011 |
|---|
| Monoisotopic Molecular
Weight: |
427.029414749 |
|---|
| InChI Key: |
CIKGWCTVFSRMJU-KVQBGUIXSA-N |
|---|
| InChI: | InChI=1S/C10H15N5O10P2/c11-10-13-8-7(9(17)14-10)12-3-15(8)6-1-4(16)5(24-6)2-23-27(21,22)25-26(18,19)20/h3-6,16H,1-2H2,(H,21,22)(H2,18,19,20)(H3,11,13,14,17)/t4-,5+,6+/m0/s1 |
|---|
| CAS
number: |
102783-74-4 |
|---|
| IUPAC Name: | [({[5-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
|
Traditional IUPAC Name: |
deoxyguanosine diphosphate |
|---|
| SMILES: | NC1=NC2=C(N=CN2[C@H]2C[C@H](O)[C@@H](COP(O)(=O)OP(O)(O)=O)O2)C(=O)N1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as purine ribonucleoside diphosphates. These are purine ribobucleotides with diphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside diphosphate
- Organic pyrophosphate
- Purine
- Imidazopyrimidine
- Hydroxypyrimidine
- Monoalkyl phosphate
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|