|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000201 |
|---|
|
Identification |
|---|
| Name: |
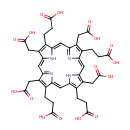
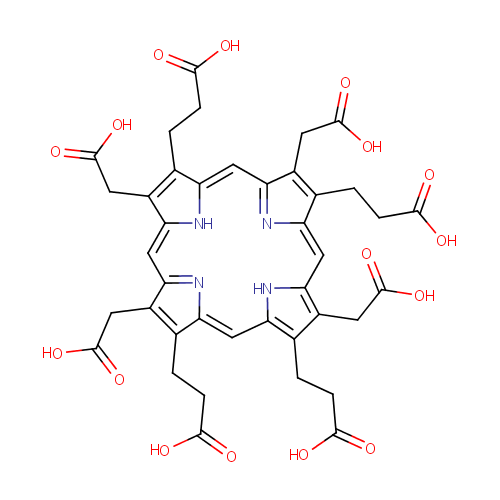
Uroporphyrin III |
|---|
| Description: | Uroporphyrin is the porphyrin produced by oxidation of the methylene bridges in uroporphyrinogen. Uroporphyrins have four acetic acid and four propionic acid side chains attached to the pyrrole rings. Uroporphyrinogen I and III are formed from polypyrryl methane in the presence of uroporphyrinogen III cosynthetase and uroporphyrin I synthetase, respectively. They can yield uroporphyrins by autooxidation or coproporphyrinogens by decarboxylation. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3,8,13,17-Tetrakis(carboxymethyl)porphyrin-2,7,12,18-tetrapropanoate
- 3,8,13,17-Tetrakis(carboxymethyl)porphyrin-2,7,12,18-tetrapropanoic acid
- 3,8,13,17-Tetramethyl-2,7,12,18-Porphinetetrapropionate
- 3,8,13,17-Tetramethyl-2,7,12,18-Porphinetetrapropionic acid
- Coproporphyrin III
- Uroporphyrin III
|
|---|
|
Chemical Formula: |
C40H38N4O16 |
|---|
| Average Molecular Weight: |
830.7469 |
|---|
| Monoisotopic Molecular
Weight: |
830.228281188 |
|---|
| InChI Key: |
VZVFNUAIRVUCEW-UJJXFSCMSA-N |
|---|
| InChI: | InChI=1S/C40H38N4O16/c45-33(46)5-1-17-21(9-37(53)54)29-14-27-19(3-7-35(49)50)22(10-38(55)56)30(43-27)15-28-20(4-8-36(51)52)24(12-40(59)60)32(44-28)16-31-23(11-39(57)58)18(2-6-34(47)48)26(42-31)13-25(17)41-29/h13-16,41,44H,1-12H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60)/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16- |
|---|
| CAS
number: |
18273-06-8 |
|---|
| IUPAC Name: | 3-[10,14,19-tris(2-carboxyethyl)-5,9,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8(23),9,11,13,15,17,19-undecaen-4-yl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
3-[10,14,19-tris(2-carboxyethyl)-5,9,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8(23),9,11,13,15,17,19-undecaen-4-yl]propanoic acid |
|---|
| SMILES: | OC(=O)CCC1=C(CC(O)=O)/C2=C/C3=N/C(=C\C4=C(CCC(O)=O)C(CC(O)=O)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(CC(O)=O)=C4CCC(O)=O)/C(CCC(O)=O)=C3CC(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Porphyrin and chlorophyll metabolism pae00860
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Bozek P, Hutta M, Hrivnakova B: Rapid analysis of porphyrins at low ng/l and microg/l levels in human urine by a gradient liquid chromatography method using octadecylsilica monolithic columns. J Chromatogr A. 2005 Aug 19;1084(1-2):24-32. Pubmed: 16114232
- Hernandez-Zavala A, Del Razo LM, Garcia-Vargas GG, Aguilar C, Borja VH, Albores A, Cebrian ME: Altered activity of heme biosynthesis pathway enzymes in individuals chronically exposed to arsenic in Mexico. Arch Toxicol. 1999 Mar;73(2):90-5. Pubmed: 10350189
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Luo J, Lim CK: Isolation and characterization of new porphyrin metabolites in human porphyria cutanea tarda and in rats treated with hexachlorobenzene by HPTLC, HPLC and liquid secondary ion mass spectrometry. Biomed Chromatogr. 1995 May-Jun;9(3):113-22. Pubmed: 7655298
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. Pubmed: 12829005
- Salen G, Berginer V, Shore V, Horak I, Horak E, Tint GS, Shefer S: Increased concentrations of cholestanol and apolipoprotein B in the cerebrospinal fluid of patients with cerebrotendinous xanthomatosis. Effect of chenodeoxycholic acid. N Engl J Med. 1987 May 14;316(20):1233-8. Pubmed: 3106810
- Schonning C, Leeming R, Stenstrom TA: Faecal contamination of source-separated human urine based on the content of faecal sterols. Water Res. 2002 Apr;36(8):1965-72. Pubmed: 12092571
- To-Figueras J, Ozalla D, Mateu CH: Long-standing changes in the urinary profile of porphyrin isomers after clinical remission of porphyria cutanea tarda. Ann Clin Lab Sci. 2003 Summer;33(3):251-6. Pubmed: 12956438
- Tsai SF, Bishop DF, Desnick RJ: Purification and properties of uroporphyrinogen III synthase from human erythrocytes. J Biol Chem. 1987 Jan 25;262(3):1268-73. Pubmed: 3805019
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Kajiwara, Masahiro; Mizutani, Minoru; Kojima, Ichiro. Manufacture of uroporphyrin III with Arthrobacter. Jpn. Kokai Tokkyo Koho (1993), 7 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|