Search Results for compounds
Searching compounds for
returned 4373 results.
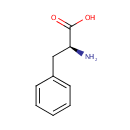
L-Phenylalanine (PAMDB000061)
IUPAC:
(2S)-2-amino-3-phenylpropanoic acid
CAS: 63-91-2
Description: Phenylalanine is an essential amino acid and the precursor for the amino acid tyrosine. Phenylalanine is a precursor of the neurotransmitters called catecholamines, which are adrenalin-like substances. Normal metabolism of phenylalanine requires biopterin, iron, niacin, vitamin B6, copper and vitamin C. Phenylalanine and tyrosine, like L-dopa, produce a catecholamine effect.
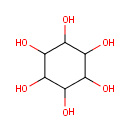
Inositol (PAMDB000062)
IUPAC:
cyclohexane-1,2,3,4,5,6-hexol
CAS: 6917-35-7
Description: Inositol or cyclohexane-1,2,3,4,5,6-hexol is a chemical compound with formula C6H12O6 or (-CHOH-)6, a six-fold alcohol (polyol) of cyclohexane. It exists in nine possible stereoisomers, of which the most prominent form, widely occuring in nature, is cis-1,2,3,5-trans-4,6-cyclohexanehexol, or myo-inositol. Other naturally occurring isomers (though in minimal quantities) are scyllo-, muco-, D-chiro-, and neo-inositol. The other possible isomers are L-chiro-, allo-, epi-, and cis-inositol. (Wikipedia)
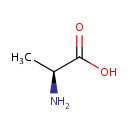
L-Alanine (PAMDB000063)
IUPAC:
(2S)-2-aminopropanoic acid
CAS: 56-41-7
Description: Alanine is an amino acid made from the conversion of the carbohydrate pyruvate or the breakdown of DNA and the dipeptides carnosine and anserine. Alanine is an important participant as well as regulator in glucose metabolism. Normal alanine metabolism, like that of other amino acids, is highly dependent upon enzymes that contain vitamin B6. (http://www.dcnutrition.com/AminoAcids/)
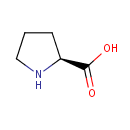
L-Proline (PAMDB000064)
IUPAC:
(2S)-pyrrolidine-2-carboxylic acid
CAS: 147-85-3
Description: L-Proline is one of the twenty amino acids used in living organisms as the building blocks of proteins. Proline is sometimes called an imino acid, although the IUPAC definition of an imine requires a carbon-nitrogen double bond. Proline is a non-essential amino acid that is synthesized from glutamic acid. It is an essential component of collagen and is important for proper functioning of joints and tendons.
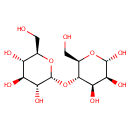
D-Maltose (PAMDB000065)
IUPAC:
(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-{[(2R,3S,4R,5S,6S)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}oxane-3,4,5-triol
CAS: 69-79-4
Description: Maltose, or malt sugar, is a disaccharide formed from two units of glucose joined with an alpha (1->4) linkage. It is the second member of an important biochemical series of glucose chains. The addition of another glucose unit yields maltotriose, Further additions will produce dextrins, also called maltodextrins, and eventually starch. Maltose can be broken down into two glucose molecules by hydrolysis in living organisms.
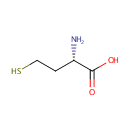
L-Homocysteine (PAMDB000067)
IUPAC:
(2S)-2-amino-4-sulfanylbutanoic acid
CAS: 6027-13-0
Description: Homocysteine is a thiol-containing amino acid formed by a demethylation of methionine. Homocysteine is a variant of the amino acid cysteine, differing in that its side-chain contains an additional methylene (-CH2-) group before the thiol (-SH) group. In Pseudomonas aeruginosa homocysteine (Hcy) is the last intermediate on the methionine biosynthetic pathway.
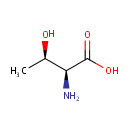
L-Threonine (PAMDB000068)
IUPAC:
(2S,3R)-2-amino-3-hydroxybutanoic acid
CAS: 72-19-5
Description: Threonine is an amino acid. It is converted to pyruvate via threonine dehydrogenase. An intermediate in this pathway can undergo thiolysis with CoA to produce acetyl-CoA and glycine. (Wikipedia)
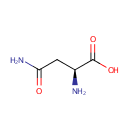
L-Asparagine (PAMDB000069)
IUPAC:
(2S)-2-amino-3-carbamoylpropanoic acid
CAS: 70-47-3
Description: Asparagine (Asn) is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side chain's functional group. It is considered a non-essential amino acid. The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing alpha-ketoglutarate and aspartate. The enzyme asparagine synthetase produces asparagine, AMP, glutamate, and pyrophosphate from aspartate, glutamine, and ATP. In the asparagine synthetase reaction, ATP is used to activate aspartate, forming beta-aspartyl-AMP. glutamine donates an ammonium group which reacts with beta-aspartyl-AMP to form asparagine and free AMP. Since the asparagine side chain can make efficient hydrogen bond interactions with the peptide backbone, asparagines are often found near the beginning and end of alpha-helices, and in turn motifs in beta sheets. Its role can be thought as "capping" the hydrogen bond interactions which would otherwise need to be satisfied by the polypeptide backbone. Glutamines have an extra methylene group, have more conformational entropy and thus are less useful in this regard. Asparagine also provides key sites for N-linked glycosylation, modification of the protein chain with the addition of carbohydrate chains. (http://en.wikipedia.org/wiki/Asparagine)
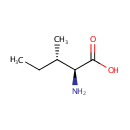
L-Isoleucine (PAMDB000071)
IUPAC:
(2S,3S)-2-amino-3-methylpentanoic acid
CAS: 73-32-5
Description: Isoleucine is an amino acid with the chemical formula HO2CCH(NH2)CH(CH3)CH2CH3. Its codons are AUU, AUC and AUA. With a hydrocarbon side chain, isoleucine is classified as a hydrophobic amino acid. Together with threonine, isoleucine is one of two common amino acids that have a chiral side chain. Four stereoisomers of isoleucine are possible, including two possible diastereomers of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid. (Wikipedia)
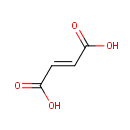
Fumaric acid (PAMDB000073)
IUPAC:
(2E)-but-2-enedioic acid
CAS: 110-17-8
Description: A precursor to L-malate in the Krebs tricarboxylic acid cycle. It is formed by the oxidation of succinate by succinate dehydrogenase. Fumarate is converted by fumarase to malate. A fumarate is a salt or ester of the organic compound fumaric acid, a dicarboxylic acid. (wikipedia)