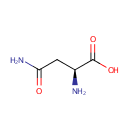
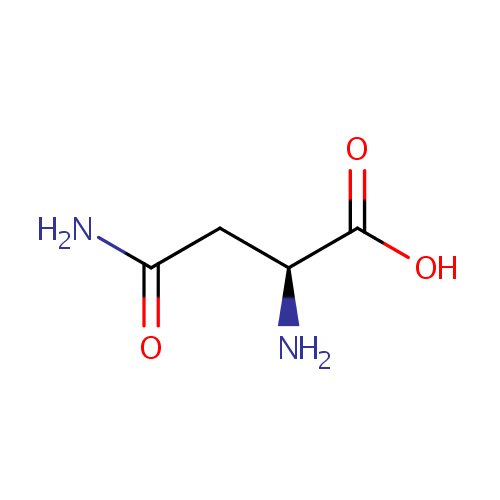
L-Asparagine (PAMDB000069)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000069 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | L-Asparagine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Asparagine (Asn) is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side chain's functional group. It is considered a non-essential amino acid. The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing alpha-ketoglutarate and aspartate. The enzyme asparagine synthetase produces asparagine, AMP, glutamate, and pyrophosphate from aspartate, glutamine, and ATP. In the asparagine synthetase reaction, ATP is used to activate aspartate, forming beta-aspartyl-AMP. glutamine donates an ammonium group which reacts with beta-aspartyl-AMP to form asparagine and free AMP. Since the asparagine side chain can make efficient hydrogen bond interactions with the peptide backbone, asparagines are often found near the beginning and end of alpha-helices, and in turn motifs in beta sheets. Its role can be thought as "capping" the hydrogen bond interactions which would otherwise need to be satisfied by the polypeptide backbone. Glutamines have an extra methylene group, have more conformational entropy and thus are less useful in this regard. Asparagine also provides key sites for N-linked glycosylation, modification of the protein chain with the addition of carbohydrate chains. (http://en.wikipedia.org/wiki/Asparagine) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C4H8N2O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 132.1179 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 132.053492132 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | DCXYFEDJOCDNAF-REOHCLBHSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C4H8N2O3/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H2,6,7)(H,8,9)/t2-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 70-47-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-amino-3-carbamoylpropanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | L-asparagine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | N[C@@H](CC(N)=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | L-alpha-amino acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 234-235 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Asparagine + Water > L-Aspartic acid + Ammonium L-Aspartic acid + Adenosine triphosphate + L-Glutamine + Water > Adenosine monophosphate + L-Asparagine + L-Glutamate + Hydrogen ion + Pyrophosphate L-Asparagine + Adenosine triphosphate + tRNA(Asn) + tRNA(Asn) <> Adenosine monophosphate + L-Asparaginyl-tRNA(Asn) + Pyrophosphate + L-Asparaginyl-tRNA(Asn) L-Aspartic acid + Adenosine triphosphate + Ammonium > Adenosine monophosphate + L-Asparagine + Hydrogen ion + Pyrophosphate Adenosine triphosphate + L-Aspartic acid + Ammonia <> Adenosine monophosphate + Pyrophosphate + L-Asparagine L-Asparagine + Water <> L-Aspartic acid + Ammonia Adenosine triphosphate + L-Aspartic acid + L-Glutamine + Water <> Adenosine monophosphate + Pyrophosphate + L-Asparagine + L-Glutamate Adenosine triphosphate + L-Asparagine + tRNA(Asn) <> Adenosine monophosphate + Pyrophosphate + L-Asparaginyl-tRNA(Asn) L-Asparagine + Water > Hydrogen ion + L-Aspartic acid + Ammonia gly-asn + Water > Glycine + L-Asparagine Adenosine triphosphate + L-Aspartic acid + L-Glutamine + Water + Ammonia <> Adenosine monophosphate + Pyrophosphate + L-Asparagine + L-Glutamate Adenosine triphosphate + L-Aspartic acid + Ammonia + L-Aspartic acid > Adenosine monophosphate + L-Asparagine + Pyrophosphate + L-Asparagine Adenosine triphosphate + L-Aspartic acid + L-Glutamine + Water + L-Aspartic acid > Adenosine monophosphate + Pyrophosphate + L-Asparagine + L-Glutamic acid + L-Asparagine + L-Glutamate L-Asparagine + Adenosine triphosphate + Hydrogen ion + tRNA(Asn) + L-Asparagine > Pyrophosphate + Adenosine monophosphate + L-asparaginyl-tRNA(Asn) L-Aspartic acid + Water + Adenosine triphosphate + L-Glutamine + L-Aspartic acid > L-Asparagine + Hydrogen ion + Adenosine monophosphate + L-Glutamic acid + Pyrophosphate + L-Asparagine + L-Glutamate L-Aspartic acid + Adenosine triphosphate + Ammonium + L-Aspartic acid > L-Asparagine + Adenosine monophosphate + Pyrophosphate + Hydrogen ion + L-Asparagine L-Asparagine + Water + L-Asparagine > L-Aspartic acid + Ammonium + L-Aspartic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Wang, Fangda. Preparation of L-b-asparagine. Faming Zhuanli Shenqing Gongkai Shuomingshu (2005), 8 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in asparaginase activity
- Specific function:
- L-asparagine + H(2)O = L-aspartate + NH(3)
- Gene Name:
- ansB
- Locus Tag:
- PA1337
- Molecular weight:
- 38.6 kDa
Reactions
| L-asparagine + H(2)O = L-aspartate + NH(3). |
- General function:
- Involved in asparaginase activity
- Specific function:
- L-asparagine + H(2)O = L-aspartate + NH(3)
- Gene Name:
- ansA
- Locus Tag:
- PA2253
- Molecular weight:
- 34.8 kDa
Reactions
| L-asparagine + H(2)O = L-aspartate + NH(3). |
Transporters
- General function:
- Involved in nucleotide binding
- Specific function:
- Probably part of a binding-protein-dependent transport system yecCS for an amino acid. Probably responsible for energy coupling to the transport system
- Gene Name:
- yecC
- Locus Tag:
- PA5152
- Molecular weight:
- 28.4 kDa