Search Results for compounds
Searching compounds for
returned 4373 results.
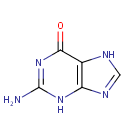
Guanine (PAMDB000051)
IUPAC:
2-amino-6,7-dihydro-3H-purin-6-one
CAS: 73-40-5
Description: Guanine is one of the five main nucleobases found in the nucleic acids DNA and RNA. Guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. Being unsaturated, the bicyclic molecule is planar. The guanine nucleoside is called guanosine. High affinity binding of guanine nucleotides and the ability to hydrolyze bound GTP to GDP are characteristics of an extended family of intracellular proteins. Hypoxanthine-guanine phosphoribosyltransferase (HPRT, EC 2.4.2.8) is a purine salvage enzyme that catalyses the conversion of hypoxanthine and guanine to their respective mononucleotides. Peroxynitrite induces DNA base damage predominantly at guanine (G) and 8-oxoguanine (8-oxoG) nucleobases via oxidation reactions. G and 8-oxoG are the most reactive bases toward Peroxynitrite and possibly the major contributors to peroxynitrite-derived genotoxic and mutagenic lesions. The neutral G radical, reacts with NO2 to yield 8-nitroguanine and 5-nitro-4-guanidinohydantoin. (PMID: 16352449, 2435586)
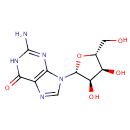
Guanosine (PAMDB000052)
IUPAC:
2-amino-9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-1H-purin-6-one
CAS: 118-00-3
Description: Guanosine is a nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a beta-N9-glycosidic bond. Guanosine can be phosphorylated to become GMP (guanosine monophosphate), cGMP (cyclic guanosine monophosphate), GDP (guanosine diphosphate) and GTP (guanosine triphosphate). (Wikipedia)
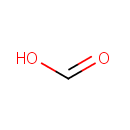
Formic acid (PAMDB000053)
IUPAC:
formic acid
CAS: 64-18-6
Description: Formic acid is the simplest carboxylic acid. Formate is an intermediate in normal metabolism. It takes part in the metabolism of one-carbon compounds and its carbon may appear in methyl groups undergoing transmethylation. It is eventually oxidized to carbon dioxide. In nature, formic acid is found in the stings and bites of many insects of the order Hymenoptera, including bees and ants. The principal use of formic acid is as a preservative and antibacterial agent in livestock feed. When sprayed on fresh hay or other silage, it arrests certain decay processes and causes the feed to retain its nutritive value longer.
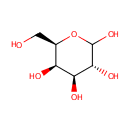
D-Galactose (PAMDB000054)
IUPAC:
(3R,4S,5R,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol
CAS: 59-23-4
Description: D-Galactose is an aldohexose that occurs naturally in the D-form in lactose, cerebrosides, gangliosides, and mucoproteins. D-Galactose is an energy-providing nutrient and also a necessary basic substrate for the biosynthesis of many macromolecules. Metabolic pathways for D-Galactose are important not only for the provision of these pathways but also for the prevention of D-Galactose and D-Galactose metabolite accumulation. The main source of D-Galactose is lactose in the milk of mammals, but it can also be found in some fruits and vegetables. Utilization of D-Galactose in all living cells is initiated by the phosphorylation of the hexose by the enzyme galactokinase (E.C. 2.7.1.6) (GALK) to form D-Galactose-1-phosphate. In the presence of D-Galactose-1-phosphate uridyltransferase (E.C. 2.7.7.12) (GALT) D-Galactose-1-phosphate is exchanged with glucose-1-phosphate in UDP-glucose to form UDP-galactose. Glucose-1-phosphate will then enter the glycolytic pathway for energy production. (PMID: 15256214, 11020650, 10408771)
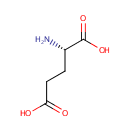
L-Glutamate (PAMDB000055)
IUPAC:
(2S)-2-aminopentanedioic acid
CAS: 56-86-0
Description: Glutamic acid (Glu), also referred to as glutamate (the anion), is one of the 20 proteinogenic amino acids. Glutamate is a key molecule in cellular metabolism. There are two forms of glutamic acid found in nature: L-glutamic acid and D-glutamic acid. D-glutamic acid is found naturally primarily in the cell walls of certain bacteria. The carboxylate anions and salts of glutamic acid are known as glutamates. Glutamate is a key compound in cellular metabolism. Glutamic acid is often used as a food additive and flavour enhancer in the form of its sodium salt monosodium glutamate (MSG). Transamination of ??-ketoglutarate gives glutamate. Glutamate will also go through transamination reactions with other ??-ketoacids.
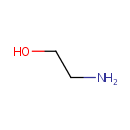
Ethanolamine (PAMDB000056)
IUPAC:
2-aminoethan-1-ol
CAS: 141-43-5
Description: Ethanolamine is a viscous, hygroscopic amino alcohol with an ammoniacal odor. Pseudomonas aeruginosa can utilize ethanolamine as the sole source of nitrogen and carbon in the presence of external vitamin B12. Ethanolamine is converted to ammonia and acetaldehyde via ethanolamin ammonia lyase. Further catabolism of acetaldehyde may be accomplished by a CoA-dependent aldehyde dehydrogenase via acetyl-CoA
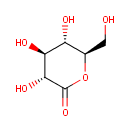
Gluconolactone (PAMDB000057)
IUPAC:
(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-one
CAS: 90-80-2
Description: Gluconolactone is a lactone or oxidized derivative of glucose. Gluconolactone is a polyhydroxy acid (PHA) that is capable of chelating metals and may also function by scavenging free radicals. When dissolved in water, it is partially hydrolysed to gluconic acid, with the balance between the lactone form and the acid form established as a chemical equilibrium. The rate of hydrolysis of gluconolactone is increased by heat and high pH. It is an intermediate in glucose and glucose-1-phosphate degradation.
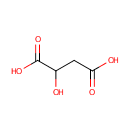
L-Malic acid (PAMDB000058)
IUPAC:
2-hydroxybutanedioic acid
CAS: 97-67-6
Description: Malic acid is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. In its ionized form it is malate, an intermediate of the TCA cycle along with fumarate. It can also be formed from pyruvate as one of the anaplerotic reactions. Apples contain malic acid, which contributes to the sourness of a green apple. Malic acid can make a wine taste tart, although the amount decreases with increasing fruit ripeness. (wikipedia)
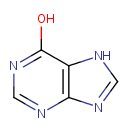
Hypoxanthine (PAMDB000059)
IUPAC:
7H-purin-6-ol
CAS: 68-94-0
Description: Hypoxanthine is a naturally occurring purine derivative and a reaction intermediate in the metabolism of adenosine and in the formation of nucleic acids by the salvage pathway. Hypoxanthine is also a spontaneous deamination product of adenine.
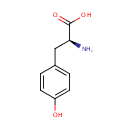
L-Tyrosine (PAMDB000060)
IUPAC:
(2S)-2-amino-3-(4-hydroxyphenyl)propanoic acid
CAS: 60-18-4
Description: Tyrosine (Tyr, Y) or 4-hydroxyphenylalanine is a non-essential amino acid with a polar side group. Its codons are UAC and UAU. Aside from being a proteogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes. It functions as a receiver of phosphate groups that are transferred by way of protein kinases (so-called receptor tyrosine kinases). Phosphorylation of the hydroxyl group changes the activity of the target protein. (Wikipedia) L-Tyrosine is the enantiomer of tyrosine (the other being D-tyrosine) that is used in building proteins.