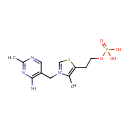Search Results for compounds
Searching compounds for
returned 4373 results.
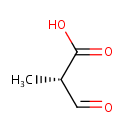
(S)-Methylmalonic acid semialdehyde (PAMDB000448)
IUPAC:
(2S)-2-methyl-3-oxopropanoic acid
CAS: 99043-16-0
Description: Methylmalonic semialdehyde is a metabolite in valine catabolism, inositol metabolism and propanoate metabolism. Methylmalonate-semialdehyde dehydrogenase (MMSDH) catalyses the NAD+ and coenzyme A-dependent conversion of methylmalonate semialdehyde to propionyl-CoA in the distal region of the L-valine catabolic pathway. Direct enzymatic assay of MMSDH is difficult since the substrate, methylmalonate semialdehyde, is both commercially unavailable and notoriously unstable as a b-keto acid.
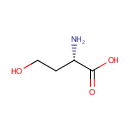
L-Homoserine (PAMDB000449)
IUPAC:
(2S)-2-amino-4-hydroxybutanoic acid
CAS: 672-15-1
Description: Homoserine is a more reactive variant of the amino acid serine. In this variant, the hydroxyl side chain contains an additional CH2 group which brings the hydroxyl group closer to its own carboxyl group, allowing it to chemically react to form a five-membered ring. This occurs at the point that amino acids normally join to their neighbours in a peptide bond.
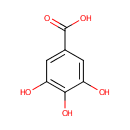
Gallic acid (PAMDB000452)
IUPAC:
3,4,5-trihydroxybenzoic acid
CAS: 149-91-7
Description: Gallic acid (GA) is an organic acid, also known as 3,4,5-trihydroxybenzoic acid, found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. Plants, bacteria and fungi are capable of synthesizing gallic acid. Shikimate dehydrogenase (SDH), a shikimate pathway enzyme essential for aromatic amino acid synthesis, is required for gallic acid production in Pseudomonas aeruginosa. SDH catalyzes NADPH-dependent reduction of 3-DHS (3-dehydroshikimate) to shikimic acid (SA), which is ultimately used to produce the aromatic amino acids l-tyrosine, l-tryptophan, and l-phenylalanine. SDH also catalyzes NADP+-dependent dehydrogenation of SA to 3-DHS, but also the dehydrogenation of 3-DHS to GA.
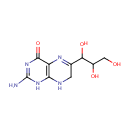
7,8-Dihydroneopterin (PAMDB000454)
IUPAC:
2-amino-6-(1,2,3-trihydroxypropyl)-1,4,7,8-tetrahydropteridin-4-one
CAS: 1218-98-0
Description: 7,8-dihydroneopterin (H(2)Neo) is an intermediate in folate biosynthesis pathway. It is converted to glycolaldehyde and 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine via bifunctional dihydroneopterin aldolase/dihydroneopterin triphosphate 2'-epimerase (EC:4.1.2.25). it is also a substrate of alkaline phosphatase. (KEGG) Dihydromonapterin is a member of the chemical class known as Biopterins and Derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. Dihydromonapterin is an intermediate in the synthesis of tetrahydromonopterin. Tetrahydromonapterin is the major tetrahydropterin in Pseudomonas aeruginosa, although the biological role of tetrahydromonapterin in Pseudomonas aeruginosa is currently unknown.
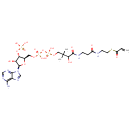
Acrylyl-CoA (PAMDB000455)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-2,2-dimethyl-3-[(2-{[2-(prop-2-enoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 5776-58-9
Description: Acrylyl-CoA is involved in alternative pathways of propionate metabolism.
Cadaverine (PAMDB000457)
IUPAC:
pentane-1,5-diamine
CAS: 462-94-2
Description: Cadaverine is a foul-smelling diamine formed by bacterial decarboxylation of lysine that occurs during protein hydrolysis during putrefaction of animal tissue. However, this diamine is not purely associated with putrefaction. It is also produced in small quantities by mammals. Cadaverine is toxic in large doses. In rats it had a low acute oral toxicity of more than 2000 mg/kg body weight (Wikipedia).
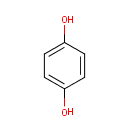
Hydroquinone (PAMDB000459)
IUPAC:
benzene-1,4-diol
CAS: 123-31-9
Description: Hydroquinone, also benzene-1,4-diol, is an aromatic organic compound which is a type of phenol, having the chemical formula C6H4(OH)2. Its chemical structure has two hydroxyl groups bonded to a benzene ring in a para position. Hydroquinone is a white granular solid at room temperature and pressure. The hydroxyl groups of hydroquinone are quite weakly acidic. Hydroquinone can lose an H+ from one of the hydroxyls to form a monophenolate ion or lose an H+ from both to form a diphenolate ion. Hydroquinone has a variety of uses principally associated with its action as a reducing agent which is soluble in water. The presence of hydroquinone in Pseudomonas aeruginosa arises from the catabolism of tyrosine and other similar aromatic substrates.
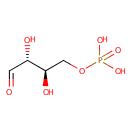
D-Erythrose 4-phosphate (PAMDB000460)
IUPAC:
[(2R,3R)-2,3-dihydroxy-4-oxobutoxy]phosphonic acid
CAS: 585-18-2
Description: D-Erythrose 4-phosphate is a phosphorylated derivative of erythrose that serves as an important intermediate in the pentose phosphate pathway. It is also used in phenylalanine, tyrosine and tryptophan biosynthesis, and it plays a role in vitamin B6 metabolism (KEGG)
Thiamine monophosphate (PAMDB000462)
IUPAC:
3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-5-[2-(phosphonooxy)ethyl]-1,3-thiazol-3-ium
CAS: 495-23-8
Description: Thiamine dihydrogen phosphate ester. The monophosphate ester of thiamine. Synonyms: monophosphothiamine; vitamin B1 monophosphate. -- Pubchem
Cysteic acid (PAMDB000464)
IUPAC:
2-amino-3-sulfopropanoic acid
CAS: 498-40-8
Description: Cysteic acid is an amino acid formed in the oxidation of cysteine; it is a precursor of taurine.