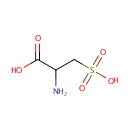
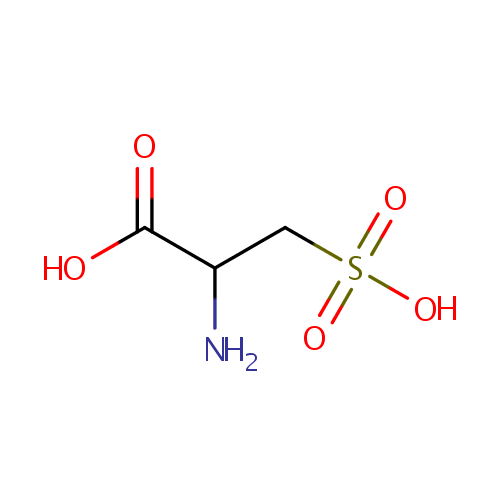
| References: |
- Jasin HE: Oxidative modification of inflammatory synovial fluid immunoglobulin G. Inflammation. 1993 Apr;17(2):167-81. Pubmed: 8387962
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Paroni R, De Vecchi E, Fermo I, Arcelloni C, Diomede L, Magni F, Bonini PA: Total urinary hydroxyproline determined with rapid and simple high-performance liquid chromatography. Clin Chem. 1992 Mar;38(3):407-11. Pubmed: 1547560
- Sarwar G, Botting HG, Peace RW: Complete amino acid analysis in hydrolysates of foods and feces by liquid chromatography of precolumn phenylisothiocyanate derivatives. J Assoc Off Anal Chem. 1988 Nov-Dec;71(6):1172-5. Pubmed: 3240976
|
|---|