|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000448 |
|---|
|
Identification |
|---|
| Name: |
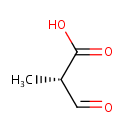
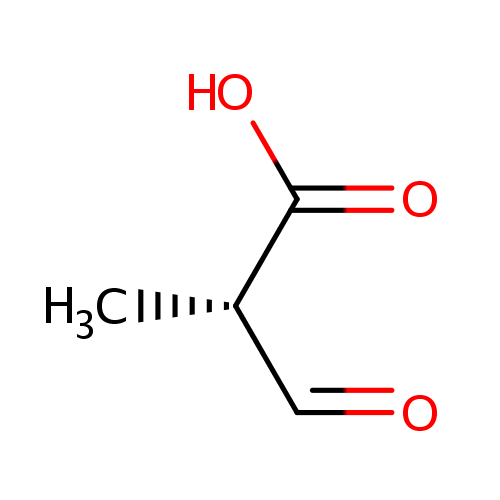
(S)-Methylmalonic acid semialdehyde |
|---|
| Description: | Methylmalonic semialdehyde is a metabolite in valine catabolism, inositol metabolism and propanoate metabolism. Methylmalonate-semialdehyde dehydrogenase (MMSDH) catalyses the NAD+ and coenzyme A-dependent conversion of methylmalonate semialdehyde to propionyl-CoA in the distal region of the L-valine catabolic pathway. Direct enzymatic assay of MMSDH is difficult since the substrate, methylmalonate semialdehyde, is both commercially unavailable and notoriously unstable as a b-keto acid. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2S)-2-methyl-3-oxopropanoate
- (2S)-2-methyl-3-oxopropanoic acid
- (S)-Methylmalonate semialdehyde
|
|---|
|
Chemical Formula: |
C4H6O3 |
|---|
| Average Molecular Weight: |
102.0886 |
|---|
| Monoisotopic Molecular
Weight: |
102.031694058 |
|---|
| InChI Key: |
VOKUMXABRRXHAR-VKHMYHEASA-N |
|---|
| InChI: | InChI=1S/C4H6O3/c1-3(2-5)4(6)7/h2-3H,1H3,(H,6,7)/t3-/m0/s1 |
|---|
| CAS
number: |
99043-16-0 |
|---|
| IUPAC Name: | (2S)-2-methyl-3-oxopropanoic acid |
|---|
|
Traditional IUPAC Name: |
(S)-methylmalonaldehydic acid |
|---|
| SMILES: | C[C@@H](C=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 1,3-dicarbonyl compounds. These are carbonyl compounds with the generic formula O=C(R)C(H)C(R')=O, where R and R' can be any group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbonyl compounds |
|---|
| Sub Class | 1,3-dicarbonyl compounds |
|---|
|
Direct Parent |
1,3-dicarbonyl compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1,3-dicarbonyl compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Short-chain aldehyde
- Aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Microbial metabolism in diverse environments pae01120
- Propanoate metabolism pae00640
- Trinitrotoluene degradation pae00633
- Valine, leucine and isoleucine degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Chambliss, K. L., Gray, R. G., Rylance, G., Pollitt, R. J., Gibson, K. M. (2000). "Molecular characterization of methylmalonate semialdehyde dehydrogenase deficiency." J Inherit Metab Dis 23:497-504. Pubmed: 10947204
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Manning NJ, Pollitt RJ: Tracer studies of the interconversion of R- and S-methylmalonic semialdehydes in man. Biochem J. 1985 Oct 15;231(2):481-4. Pubmed: 4062908
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|