Search Results for compounds
Searching compounds for
returned 4373 results.
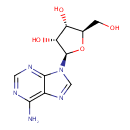
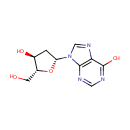
Adenosine (PAMDB000016)
IUPAC:
(2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
CAS: 58-61-7
Description: Adenosine is nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter.
Ammonia (PAMDB000017)
IUPAC:
ammonia
CAS: 7664-41-7
Description: Ammonia is a colorless alkaline gas with a characteristic sharp smell. Ammonia is one of the most abundant nitrogen-containing compounds in the atmosphere. It is an irritant with a characteristic pungent odor, which is widely used in industry. Because of its many uses, ammonia is one of the most highly produced inorganic chemicals.
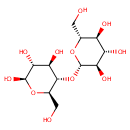
Cellobiose (PAMDB000018)
IUPAC:
(2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}oxane-3,4,5-triol
CAS: 528-50-7
Description: Cellobiose is a disaccharide consisting of two glucose units in a beta (1-4) glycosidic linkage. It is an intermediate of starch and sucrose metabolism. It is formed through the action of endo-1,4-D-glucanase and converted to beta-D-glucose by beta-D-glucoside glucohydrolase (EC:3.2.1.21). (KEGG)
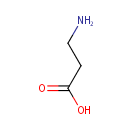
beta-Alanine (PAMDB000019)
IUPAC:
3-aminopropanoic acid
CAS: 107-95-9
Description: Beta-alanine is the only naturally occurring beta-amino acid. However, it is not used in the biosynthesis of any major proteins or enzymes. It is formed in vivo by the degradation of dihydrouracil and carnosine. It is a component of pantothenic acid (vitamin B5), which itself is a component of coenzyme A. Under normal conditions, beta-alanine is metabolized into acetic acid.
Cyclic AMP (PAMDB000020)
IUPAC:
(4aR,6R,7R,7aS)-6-(6-amino-9H-purin-9-yl)-2,7-dihydroxy-hexahydro-2???furo[3,2-d][1,3,2]dioxaphosphinin-2-one
CAS: 60-92-4
Description: Cyclic AMP is an adenine nucleotide containing one phosphate group which is esterified to both the 3'- and 5'-positions of the sugar moiety. It is a second messenger and a key intracellular regulator. cAMP is synthesized from ATP by adenylate cyclase. Adenylate cyclase is located at the cell membranes. cAMP decomposition into AMP is catalyzed by the enzyme phosphodiesterase.
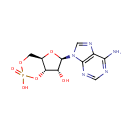
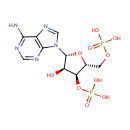
Adenosine 3',5'-diphosphate (PAMDB000022)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}phosphonic acid
CAS: 1053-73-2
Description: Adenosine 3', 5'-diphosphate or PAP is a nucleotide that is closely related to ADP. It has two phosphate groups attached to the 5' and 3' positions of the pentose sugar ribose (instead of pyrophosphoric acid at the 5' position, as found in ADP), and the nucleobase adenine. PAP is converted to 3'-phosphoadenosine 5'-phosphosulfate (PAPS) by Sulfotransferase and then back to PAP after the sulfotransferase reaction. Sulfotransferase (STs) catalyze the transfer reaction of the sulfate group from the ubiquitous donor 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to an acceptor group of numerous substrates. This reaction, often referred to as sulfuryl transfer, sulfation, or sulfonation plays a key role in various biological processes such as cell communication, growth and development, and defense. (HMDB) PAP is involved in sulfur metabolism in Pseudomonas aeruginosa, and can be converted into AMP and subsequently ADP by enzymes. (PMID 10939241)
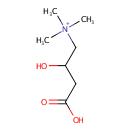
Carnitine (PAMDB000023)
IUPAC:
(3-carboxy-2-hydroxypropyl)trimethylazanium
CAS: 541-15-1
Description: Carnitine is a quaternary ammonium compound biosynthesized from the amino acids lysine and methionine. In living cells, it is required for the transport of fatty acids during the breakdown of lipids (fats) for the generation of metabolic energy. Carnitine was originally found as a growth factor for mealworms and labeled vitamin Bt. Carnitine exists in two stereoisomers: Its biologically active form is L-carnitine, whereas its enantiomer, D-carnitine, is biologically inactive. Pseudomonas aeruginosa converts carnitine, via crotonobetaine, to gamma-butyrobetaine in the presence of C and N sources and under anaerobic conditions. This two-step pathway requires L-(-)-carnitine dehydratase and crotonobetaine reductase. Anaerobic carnitine metabolism in Pseudomonas aeruginosa involves six genes organized in the cai operon and located at the first minute on the Pseudomonas aeruginosa chromosome.
Deoxyinosine (PAMDB000024)
IUPAC:
9-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-9H-purin-6-ol
CAS: 890-38-0
Description: Deoxyinosine is a nucleoside that is formed when hypoxanthine is attached to a deoxyribose ring (also known as a ribofuranose) via a beta-N9-glycosidic bond. Deoxyinosine is found in DNA while Inosine is found in RNA. Inosine is a nucleic acid important for RNA editing. Adenosine deaminase (ADA) catalyzes the conversion of adenosine and deoxyadenosine to inosine and deoxyinosine, respectively.
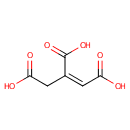
cis-Aconitic acid (PAMDB000025)
IUPAC:
(1Z)-prop-1-ene-1,2,3-tricarboxylic acid
CAS: 585-84-2
Description: cis-Aconitic acid is an intermediate in the tricarboxylic acid cycle produced by the dehydration of citric acid. The enzyme aconitase (aconitate hydratase; EC 4.2.1.3) catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle
Cysteinylglycine (PAMDB000028)
IUPAC:
2-[(2R)-2-amino-3-sulfanylpropanamido]acetic acid
CAS: 19246-18-5
Description: Cysteinylglycine is a naturally occurring dipeptide. It is derived from the breakdown of glutathione (a tripeptide).