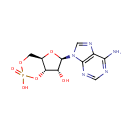
| Synonyms: | - 3',5'-Cyclic AMP
- 3'5'-Cyclic AMP
- 6-(6-Amino-9H-purin-9-yl)tetrahydro-4H-furo[3,2-D][1,3,2]dioxaphosphinine-2,7-diol 2-oxide
- Acrasin
- Adenosine 3',5'-cyclic monophosphate
- Adenosine 3',5'-cyclic monophosphoric acid
- Adenosine 3',5'-cyclic phosphate
- Adenosine 3',5'-cyclic phosphoric acid
- Adenosine 3',5'-cyclophosphate
- Adenosine 3',5'-cyclophosphoric acid
- Adenosine 3',5'-monophosphate
- Adenosine 3',5'-monophosphoric acid
- Adenosine 3,5'-cyclic monophosphate
- Adenosine 3,5'-cyclic monophosphorate
- Adenosine 3,5'-cyclic monophosphoric acid
- Adenosine cyclic monophosphate
- Adenosine cyclic monophosphoric acid
- Adenosine cyclic-3',5'-monophosphate
- Adenosine cyclic-3',5'-monophosphoric acid
- Adenosine cyclic-monophosphate
- Adenosine cyclic-monophosphoric acid
- Adenosine-3',5'-cyclic monophosphate
- Adenosine-3',5'-cyclic monophosphoric acid
- Adenosine-3',5'-monophosphate
- Adenosine-3',5'-monophosphoric acid
- Adenosine-cyclic-phosphate
- Adenosine-cyclic-phosphoric acid
- Adenosine-cyclic-phosphoric-acid
- CAMP
- Cyclic 3',5'-adenylate
- Cyclic 3',5'-adenylic acid
- Cyclic 3',5'-AMP
- Cyclic adenosine 3',5'-phosphate
- Cyclic adenosine 3',5'-phosphoric acid
- Cyclic AMP
|
|---|
| References: |
- Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J., Rabinowitz, J. D. (2009). "Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli." Nat Chem Biol 5:593-599. Pubmed: 19561621
- Carceles MD, Ribo AR, Davalos R, Martinez T, Hernandez J: Effect of diazepam on adenosine 3',5'-cyclic monophosphate (cAMP) plasma levels in anesthetized patients. Clin Ther. 2004 May;26(5):737-43. Pubmed: 15220017
- Chu MS, Chang CF, Yang CC, Bau YC, Ho LL, Hung SC: Signalling pathway in the induction of neurite outgrowth in human mesenchymal stem cells. Cell Signal. 2006 Apr;18(4):519-30. Epub 2005 Aug 11. Pubmed: 16098715
- Fischer JA, Bourne HR, Dambacher MA, Tschopp F, De Meyer R, Devogelaer JP, Werder EA, Nagant De Deuxchaisnes C: Pseudohypoparathyroidism: inheritance and expression of deficient receptor-cyclase coupling protein activity. Clin Endocrinol (Oxf). 1983 Dec;19(6):747-54. Pubmed: 6317236
- Fouassier L, Chinet T, Robert B, Carayon A, Balladur P, Mergey M, Paul A, Poupon R, Capeau J, Barbu V, Housset C: Endothelin-1 is synthesized and inhibits cyclic adenosine monophosphate- dependent anion secretion by an autocrine/paracrine mechanism in gallbladder epithelial cells. J Clin Invest. 1998 Jun 15;101(12):2881-8. Pubmed: 9637723
- Imashuku S, Todo S, Nakajima F: [Intra-aortic prostaglandin E1 infusion in the treatment of advanced neuroblastoma] Gan To Kagaku Ryoho. 1983 Sep;10(9):1936-43. Pubmed: 6311112
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kukreja SC, Shevrin DH, Wimbiscus SA, Ebeling PR, Danks JA, Rodda CP, Wood WI, Martin TJ: Antibodies to parathyroid hormone-related protein lower serum calcium in athymic mouse models of malignancy-associated hypercalcemia due to human tumors. J Clin Invest. 1988 Nov;82(5):1798-802. Pubmed: 2846659
- Lerche A, Svenson M, Wiik A: Cerebrospinal fluid levels of cyclic nucleotides in meningitis and idiopathic polyneuritis. Acta Neurol Scand. 1984 Mar;69(3):168-75. Pubmed: 6326460
- Liu H, Chang L, Chen Y, Xia S, Zhang X: Clinical implication of the changes of cAMP, TXA2 and PGI2 in CSF of asphyxiated newborns. J Huazhong Univ Sci Technolog Med Sci. 2003;23(2):195-7, 200. Pubmed: 12973949
- Machen TE: Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006 Aug;291(2):C218-30. Pubmed: 16825601
- Mashayekhi F, Aghahoseini F, Rezaie A, Zamani MJ, Khorasani R, Abdollahi M: Alteration of cyclic nucleotides levels and oxidative stress in saliva of human subjects with periodontitis. J Contemp Dent Pract. 2005 Nov 15;6(4):46-53. Pubmed: 16299606
- Naef A, Keller HU: A short transient increase in cyclic adenosine 3', 5'-monophosphate levels of neutrophil granulocytes following exposure to chemotactic factors. Adv Exp Med Biol. 1982;141:39-48. Pubmed: 6283833
- Onali P, Strada SJ, Chang L, Epstein PM, Hersh EM, Thompson WJ: Purification and characterization of high-affinity cyclic adenosine 5'-monophosphate phosphodiesterases from human acute myelogenous leukemic cells. Cancer Res. 1985 Mar;45(3):1384-91. Pubmed: 2982489
- Rademaker MT, Charles CJ, Lewis LK, Yandle TG, Cooper GJ, Coy DH, Richards AM, Nicholls MG: Beneficial hemodynamic and renal effects of adrenomedullin in an ovine model of heart failure. Circulation. 1997 Sep 16;96(6):1983-90. Pubmed: 9323090
- Rudman D, O'Brien MS, McKinney AS, Hoffman JC Jr, Patterson JH: Observations on the cyclic nucleotide concentrations in human cerebrospinal fluid. J Clin Endocrinol Metab. 1976 Jun;42(6):1088-97. Pubmed: 180045
- Ruppert D, Weithmann KU: HL 725, an extremely potent inhibitor of platelet phosphodiesterase and induced platelet aggregation in vitro. Life Sci. 1982 Nov 8;31(19):2037-43. Pubmed: 6294426
- Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, Kikuchi K, Nagi T, Harada M, Ogi K, Ebisawa M, Mori M: Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006 Mar 24;341(4):1078-87. Epub 2006 Jan 25. Pubmed: 16460680
- Tanaka Y, Horinouchi T, Koike K: New insights into beta-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin Exp Pharmacol Physiol. 2005 Jul;32(7):503-14. Pubmed: 16026507
- Watanabe K, Beinborn M, Nagamatsu S, Ishida H, Takahashi S: Menetrier's disease in a patient with Helicobacter pylori infection is linked to elevated glucagon-like peptide-2 activity. Scand J Gastroenterol. 2005 Apr;40(4):477-81. Pubmed: 16028444
- Wickenheisser JK, Nelson-DeGrave VL, McAllister JM: Human ovarian theca cells in culture. Trends Endocrinol Metab. 2006 Mar;17(2):65-71. Epub 2006 Feb 7. Pubmed: 16460956
- Wine JJ, Joo NS: Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1(1):47-53. Pubmed: 16113412
|
|---|