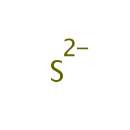Search Results for compounds
Searching compounds for
returned 4373 results.
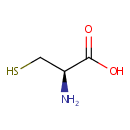
L-Cysteine (PAMDB000152)
IUPAC:
(2R)-2-amino-3-sulfanylpropanoic acid
CAS: 52-90-4
Description: Cysteine is a naturally occurring, sulfur-containing amino acid that is found in most proteins, although only in small quantities. Cysteine is unique amongst the twenty natural amino acids as it contains a thiol group. Thiol groups can undergo oxidation/reduction (redox) reactions; when cysteine is oxidized it can form cystine, which is two cysteine residues joined by a disulfide bond. This reaction is reversible: as reduction of this disulphide bond regenerates two cysteine molecules. The disulphide bonds of cystine are crucial to defining the structures of many proteins. Cysteine is often involved in electron-transfer reactions, and help the enzyme catalyze its reaction. Cysteine is also part of the antioxidant glutathione. Oxidation of cysteine can produce a disulfide bond with another thiol, or further oxidation can produce sulphfinic or sulfonic acids. The cysteine thiol group is also a nucleophile and can undergo addition and substitution reactions. Thiol groups become much more reactive when they are ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell. The thiol group also has a high affinity for heavy metals and proteins containing cysteine will bind metals such as mercury, lead and cadmium tightly.Due to this ability to undergo redox reactions, cysteine has antioxidant properties. Cysteine is important in energy metabolism. (http://www.dcnutrition.com/AminoAcids/)
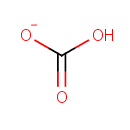
Hydrogen carbonate (PAMDB000153)
IUPAC:
hydrogen carbonate
CAS: 71-52-3
Description: Bicarbonate is a simple single carbon molecule that plays surprisingly important roles in diverse biological processes. It is an anion that consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. The bicarbonate ion carries a negative one formal charge and is the conjugate base of carbonic acid, H2CO3. The carbonate radical is an elusive and strong one-electron oxidant. Bicarbonate in equilibrium with carbon dioxide constitutes the main physiological buffer in cells. The bicarbonate-carbon dioxide pair stimulates the oxidation, peroxidation and nitration of several biological targets. The carbonate radical has also been proposed to be responsible for the stimulatory effects of the bicarbonate-carbon dioxide pair on oxidations mediated by hydrogen peroxide/transition metal ions. The ultimate precursor of the carbonate radical anion being bicarbonate, carbon dioxide, peroxymonocarbonate or complexes of transition metal ions with bicarbonate-derived species remains a matter of debate.
Sulfide (PAMDB000154)
IUPAC:
sulfanediide
CAS: 18496-25-8
Description: Sulfide is an ion form of sulfur, frequently found in hydrogen sulfide. Sulfur (Greek is theion) is the chemical element in the periodic table that has the symbol S and atomic number 16. It is an abundant, tasteless, odorless, multivalent non-metal. Sulfur, in its native form, is a yellow crystalline solid. In nature, it can be found as the pure element or as sulfide and sulfate minerals. It is an essential element for life. The amino acids cysteine and methionine contain sulfur, as do all polypeptides, proteins, and enzymes which contain these amino acids. This makes sulfur a necessary component of all living cells. Disulfide bonds between polypeptides are very important in protein assembly and structure. Homocysteine and taurine are also sulfur containing amino acids but are not coded for by DNA nor are they part of the primary structure of proteins. Some forms of bacteria use hydrogen sulfide (H2S) in the place of water as the electron donor in a primitive photosynthesis-like process. Inorganic sulfur forms a part of iron-sulfur clusters, and sulfur is the bridging ligand in the CuA site of cytochrome c oxidase. Sulfur is an important component of coenzyme A.
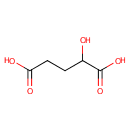
D-2-Hydroxyglutaric acid (PAMDB000155)
IUPAC:
2-hydroxypentanedioic acid
CAS: 13095-47-1
Description: D-2-Hydroxyglutaric acid is a member of the chemical class known as Short-chain Hydroxy Acids and Derivatives. These are hydroxy acids with an alkyl chain the contains less than 6 carbon atoms.
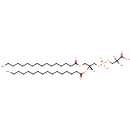
PS(16:0/16:0) (PAMDB000157)
IUPAC:
(2S)-2-amino-3-({[(2R)-2,3-bis(hexadecanoyloxy)propoxy](hydroxy)phosphoryl}oxy)propanoic acid
CAS: 3036-82-6
Description: PS(16:0/16:0) is a phosphatidylserine. It is a glycerophospholipid in which a phosphorylserine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PS(16:0/16:0), in particular, consists of two hexadecanoyl chains at positions C-1 and C-2. Phosphatidylserine or 1,2-diacyl-sn-glycero-3-phospho-L-serine is distributed widely among animals, plants and microorganisms. Phosphatidylserine is an acidic (anionic) phospholipid with three ionizable groups, i.e. the phosphate moiety, the amino group and the carboxyl function. As with other acidic lipids, it exists in nature in salt form, but it has a high propensity to chelate to calcium via the charged oxygen atoms of both the carboxyl and phosphate moieties, modifying the conformation of the polar head group. This interaction may be of considerable relevance to the biological function of phosphatidylserine. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. Phosphatidylserines typically carry a net charge of -1 at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PS biosynthesis involves an exchange reaction of serine for ethanolamine in PE.
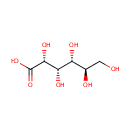
Gluconic acid (PAMDB000160)
IUPAC:
(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoic acid
CAS: 526-95-4
Description: Gluconic acid occurs naturally in fruit, honey and wine and is used as a food additive, an acidity regulator. It is also used in cleaning products where it helps cleaning up mineral deposits. It is a strong chelating agent, especially in alkaline solution. It chelates the anions of calcium, iron, aluminium, copper, and other heavy metals. Glucono delta lactone is a cyclic ester of D-gluconic acid.
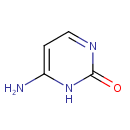
Cytosine (PAMDB000161)
IUPAC:
6-amino-1,2-dihydropyrimidin-2-one
CAS: 71-30-7
Description: Cytosine (C) is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an amine group at position 4 and a keto group at position 2). The nucleoside of cytosine is cytidine.
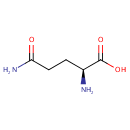
L-Glutamine (PAMDB000163)
IUPAC:
(2S)-2-amino-4-carbamoylbutanoic acid
CAS: 56-85-9
Description: Glutamine (Gln) is one of the 20 amino acids encoded by the standard genetic code. Its side chain is an amide; it is formed by replacing a side-chain hydroxyl of glutamic acid with an amine functional group. Glutamine has a variety of biochemical functions including: 1. As any other amino acid, a major role in protein synthesis. 2. Cellular energy source, next to glucose. 3. Nitrogen donor for many anabolic processes. 4.Carbon source, refilling the Citric acid cycle. (Wikipedia)
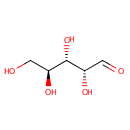
L-Arabinose (PAMDB000164)
IUPAC:
(2R,3S,4S)-2,3,4,5-tetrahydroxypentanal
CAS: 5328-37-0
Description: L-Arabinose is a pentose with a sweet taste. In Pseudomonas aeruginosa, it is involved in the catabolism of arabinose, a process directed by the L-arabinose operon. This process is activated in the presence of arabinose and the absence of glucose. (Wikipedia)
Copper (PAMDB000166)
IUPAC:
copper(2+) ion
CAS: 7440-50-8
Description: Copper is s a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; a freshly exposed surface has a reddish-orange color. The catalytic activity of copper is used by the enzymes that it is associated with and is thus only toxic when unsequestered and unmediated. Copper(II) ions are water-soluble, where they function at low concentration as bacteriostatic substances, fungicides, and wood preservatives.