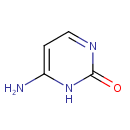
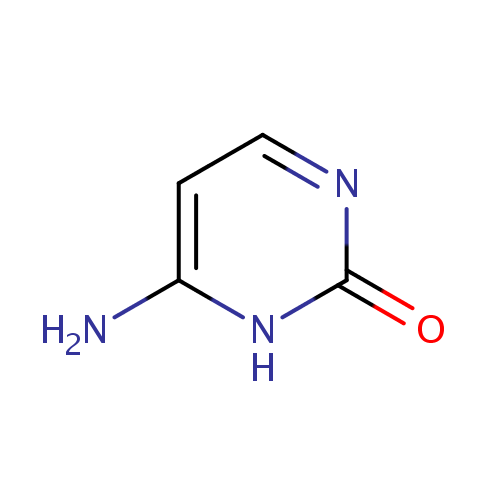
| References: |
- Bawdon RE, Sobhi S, Dax J: The transfer of anti-human immunodeficiency virus nucleoside compounds by the term human placenta. Am J Obstet Gynecol. 1992 Dec;167(6):1570-4. Pubmed: 1335207
- Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J., Rabinowitz, J. D. (2009). "Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli." Nat Chem Biol 5:593-599. Pubmed: 19561621
- Carotti D, Funiciello S, Lavia P, Caiafa P, Strom R: Different effects of histone H1 on de novo DNA methylation in vitro depend on both the DNA base composition and the DNA methyltransferase. Biochemistry. 1996 Sep 10;35(36):11660-7. Pubmed: 8794746
- Cheng JC, Yoo CB, Weisenberger DJ, Chuang J, Wozniak C, Liang G, Marquez VE, Greer S, Orntoft TF, Thykjaer T, Jones PA: Preferential response of cancer cells to zebularine. Cancer Cell. 2004 Aug;6(2):151-8. Pubmed: 15324698
- Costa E, Grayson DR, Mitchell CP, Tremolizzo L, Veldic M, Guidotti A: GABAergic cortical neuron chromatin as a putative target to treat schizophrenia vulnerability. Crit Rev Neurobiol. 2003;15(2):121-42. Pubmed: 14977367
- Harvey BG, Maroni J, O'Donoghue KA, Chu KW, Muscat JC, Pippo AL, Wright CE, Hollmann C, Wisnivesky JP, Kessler PD, Rasmussen HS, Rosengart TK, Crystal RG: Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002 Jan 1;13(1):15-63. Pubmed: 11779412
- Ishii, N., Nakahigashi, K., Baba, T., Robert, M., Soga, T., Kanai, A., Hirasawa, T., Naba, M., Hirai, K., Hoque, A., Ho, P. Y., Kakazu, Y., Sugawara, K., Igarashi, S., Harada, S., Masuda, T., Sugiyama, N., Togashi, T., Hasegawa, M., Takai, Y., Yugi, K., Arakawa, K., Iwata, N., Toya, Y., Nakayama, Y., Nishioka, T., Shimizu, K., Mori, H., Tomita, M. (2007). "Multiple high-throughput analyses monitor the response of E. coli to perturbations." Science 316:593-597. Pubmed: 17379776
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Machwe A, Orren DK, Bohr VA: Accelerated methylation of ribosomal RNA genes during the cellular senescence of Werner syndrome fibroblasts. FASEB J. 2000 Sep;14(12):1715-24. Pubmed: 10973920
- Putta MR, Zhu F, Li Y, Bhagat L, Cong Y, Kandimalla ER, Agrawal S: Novel oligodeoxynucleotide agonists of TLR9 containing N3-Me-dC or N1-Me-dG modifications. Nucleic Acids Res. 2006 Jun 23;34(11):3231-8. Print 2006. Pubmed: 16798912
- Rodriguez Ortner E, Hayes RB, Weissfeld J, Gelmann EP: Effect of homeodomain protein NKX3.1 R52C polymorphism on prostate gland size. Urology. 2006 Feb;67(2):311-5. Epub 2006 Jan 25. Pubmed: 16442598
- Sawamura D, Abe R, Goto M, Akiyama M, Hemmi H, Akira S, Shimizu H: Direct injection of plasmid DNA into the skin induces dermatitis by activation of monocytes through toll-like receptor 9. J Gene Med. 2005 May;7(5):664-71. Pubmed: 15655803
- Sigalotti L, Coral S, Nardi G, Spessotto A, Cortini E, Cattarossi I, Colizzi F, Altomonte M, Maio M: Promoter methylation controls the expression of MAGE2, 3 and 4 genes in human cutaneous melanoma. J Immunother. 2002 Jan-Feb;25(1):16-26. Pubmed: 11924907
- Tawa R, Ueno S, Yamamoto K, Yamamoto Y, Sagisaka K, Katakura R, Kayama T, Yoshimoto T, Sakurai H, Ono T: Methylated cytosine level in human liver DNA does not decline in aging process. Mech Ageing Dev. 1992 Mar 1;62(3):255-61. Pubmed: 1583911
- Thajeb P, Ma YS, Tzen CY, Chuang CK, Wu TY, Chen SC, Wei YH: Oculopharyngeal somatic myopathy in a patient with a novel large-scale 3,399 bp deletion and a homoplasmic T5814C transition of the mitochondrial DNA. Clin Neurol Neurosurg. 2006 Jun;108(4):407-10. Pubmed: 16644408
- Tsuchiya K, Tajima H, Yamada M, Takahashi H, Kuwae T, Sunaga K, Katsube N, Ishitani R: Disclosure of a pro-apoptotic glyceraldehyde-3-phosphate dehydrogenase promoter: anti-dementia drugs depress its activation in apoptosis. Life Sci. 2004 May 14;74(26):3245-58. Pubmed: 15094325
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|