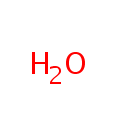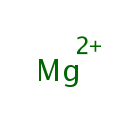Search Results for compounds
Searching compounds for
returned 4373 results.
Caprylic acid (PAMDB000140)
IUPAC:
octanoic acid
CAS: 124-07-2
Description: Caprylic acid belongs to the class of Straight Chain Fatty Acids. These are fatty acids with a straight aliphatic chain. (inferred from compound structure). Caprylic acid is the common name for the eight-carbon saturated fatty acid known by the systematic name octanoic acid. (WikiPedia). Caprylic acid is an intermediate in lipoate biosynthesis and incorporation II in Pseudomonas aeruginosa. It is a substrate for lipoate-protein ligase A which catalyzes the chemical reaction a [lipoyl-carrier protein]-L-lysine + caprylic acid + ATP -> a [lipoyl-carrier protein] N6-octanoyl-L-lysine + AMP + diphosphate + H+. It is also a substrate for acyl-ACP synthetase in the reaction caprylic acid + a holo-[acyl-carrier protein] + ATP ??an octanoyl-[acp] + AMP + diphosphate (EcoCyc compound: CPD-195).
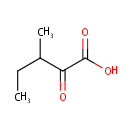
3-Methyl-2-oxovaleric acid (PAMDB000141)
IUPAC:
3-methyl-2-oxopentanoic acid
CAS: 1460-34-0
Description: 3-Methyl-2-oxovaleric acid is a metabolite of isoleucine in man, animals and bacteria. It is the alpha-keto acid analogue of isoleucine. 3-Methyl-2-oxovaleric acid is produced from isoleucine by cytosolic branched chain aminotransferase 1 (EC:2.6.1.42), whereupon it is further degraded by branched chain keto acid dehydrogenase E1 to 2-Methyl-1-hydroxybutyl-ThPP.
Water (PAMDB000142)
IUPAC:
water
CAS: 7732-18-5
Description: Water is a chemical substance that is essential to all known forms of life. It appears colorless to the naked eye in small quantities, though it is actually slightly blue in color. Water is vital both as a solvent in which many of the solutes dissolve and as an essential part of many metabolic processes within the living organisms. Metabolism is the sum total of anabolism and catabolism. In anabolism, water is removed from molecules (through energy requiring enzymatic chemical reactions) in order to grow larger molecules (e.g. starches, triglycerides and proteins for storage of fuels and information). In catabolism, water is used to break bonds in order to generate smaller molecules (e.g. glucose, fatty acids and amino acids to be used for fuels for energy use or other purposes). Water is thus essential and central to these metabolic processes..Water is also central to acid-base neutrality and enzyme function. An acid, a hydrogen ion (H+, that is, a proton) donor, can be neutralized by a base, a proton acceptor such as hydroxide ion (OH-) to form water. Water is considered to be neutral, with a pH (the negative log of the hydrogen ion concentration) of 7. Acids have pH values less than 7 while bases have values greater than 7. (Wikipedia)
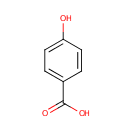
4-Hydroxybenzoic acid (PAMDB000143)
IUPAC:
4-hydroxybenzoic acid
CAS: 99-96-7
Description: 4-Hydroxybenzoic acid, or p-hydroxybenzoic acid, is a phenolic derivative of benzoic acid. It a white crystalline solid that is slightly soluble in water and chloroform, but well soluble in alcohols, ether, and acetone.
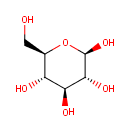
b-D-Glucose (PAMDB000145)
IUPAC:
(2R,3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol
CAS: 492-61-5
Description: B-D-glucose is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. Glucose (C6H12O6, also known as D-glucose, dextrose, or grape sugar) is a simple sugar (monosaccharide) and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate. Glucose is one of the main products of photosynthesis and fuels for cellular respiration. Glucose exists in several different molecular structures, but all of these structures can be divided into two families of mirror-images (stereoisomers). Only one set of these isomers exists in nature, those derived from the right-handed form of glucose, denoted D-glucose. D-glucose is sometimes referred to as dextrose, although the use of this name is strongly discouraged. The term dextrose is derived from dextrorotatory glucose. This name is therefore confusing when applied to the enantiomer, which rotates light the opposite direction. Starch and cellulose are polymers derived from the dehydration of D-glucose. The other stereoisomer, called L-glucose, is hardly ever found in nature. (WikiPedia)
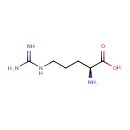
L-Arginine (PAMDB000146)
IUPAC:
(2S)-2-amino-5-carbamimidamidopentanoic acid
CAS: 74-79-3
Description: Arginine is an amino acid that is physiologically active in the L-form. Arginine can be considered to be a basic amino acid as the part of the side chain nearest to the backbone is long, carbon-containing and hydrophobic, whereas the end of the side chain is a complex guanidinium group. With a pKa of 12.48, the guanidinium group is positively charged in neutral, acidic and even most basic environments. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized. This group is able to form multiple H-bonds. The main catabolic arginine pathway of Pseudomonas aeruginosa is the succinyltransferase pathway.
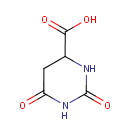
4,5-Dihydroorotic acid (PAMDB000147)
IUPAC:
2,6-dioxo-1,3-diazinane-4-carboxylic acid
CAS: 155-54-4
Description: 4,5-Dihydroorotic acid is a substrate of the enzyme orotate reductase [EC 1.3.1.14], which is part of the pyrimidine metabolism pathway. (KEGG) Dihydroorotate is oxidized by Dihydroorotate dehydrogenases (DHODs) to orotate. These dehydrogenases use their FMN (flavin mononucleotide) prosthetic group to abstract a hydride equivalent from C6 to deprotonate C5
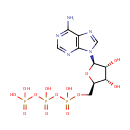
Adenosine triphosphate (PAMDB000148)
IUPAC:
({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid
CAS: 56-65-5
Description: Adenosine-5'-triphosphate (ATP) is a multifunctional nucleotide that plays an important role in cell biology as a coenzyme, that is, the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism. It is an energy source produced during photosynthesis and cellular respiration and consumed by many enzymes and a multitude of cellular processes, including biosynthetic reactions, motility, and cell division.ATP is made from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and its use in metabolism converts it back into these precursors. ATP is therefore continuously recycled in organisms. ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids, as well as by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in energy metabolism and signaling, ATP is also incorporated into nucleic acids by polymerases in the processes of DNA replication and transcription. (Wikipedia)
Magnesium (PAMDB000149)
IUPAC:
magnesium(2+) ion
CAS: 7439-95-4
Description: Magnesium is an alkaline earth metal. Due to the important interaction between phosphate and magnesium ions, magnesium ions are essential to the basic nucleic acid chemistry of life, and thus are essential to all cells of all known living organisms. Over 300 enzymes require the presence of magnesium ions for their catalytic action, including all enzymes utilizing or synthesizing ATP, or those which use other nucleotides to synthesize DNA and RNA. ATP exists in cells normally as a chelate of ATP and a magnesium ion. (Wikipedia)
Coproporphyrin III (PAMDB000151)
IUPAC:
3-[10,14,19-tris(2-carboxyethyl)-5,9,15,20-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8(23),9,11,13,15,17,19-undecaen-4-yl]propanoic acid
CAS: 14643-66-4
Description: Coproporphyrin III is a porphyrin metabolite arising from heme synthesis. Coproporphyrin III is a tetrapyrrole dead-end product from the spontaneous oxidation of the methylene bridges of coproporphynogen, arising from heme synthesis.