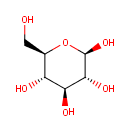
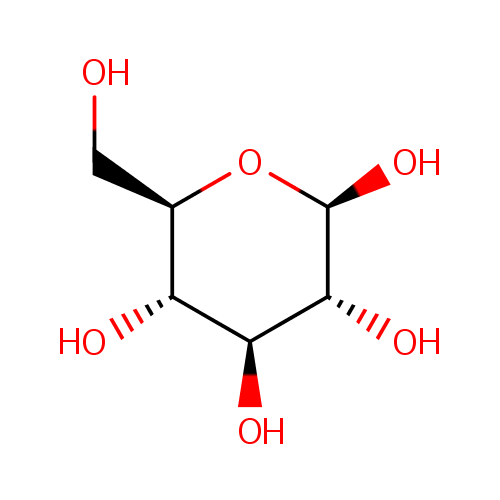
b-D-Glucose (PAMDB000145)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000145 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | b-D-Glucose | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | B-D-glucose is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. Glucose (C6H12O6, also known as D-glucose, dextrose, or grape sugar) is a simple sugar (monosaccharide) and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate. Glucose is one of the main products of photosynthesis and fuels for cellular respiration. Glucose exists in several different molecular structures, but all of these structures can be divided into two families of mirror-images (stereoisomers). Only one set of these isomers exists in nature, those derived from the right-handed form of glucose, denoted D-glucose. D-glucose is sometimes referred to as dextrose, although the use of this name is strongly discouraged. The term dextrose is derived from dextrorotatory glucose. This name is therefore confusing when applied to the enantiomer, which rotates light the opposite direction. Starch and cellulose are polymers derived from the dehydration of D-glucose. The other stereoisomer, called L-glucose, is hardly ever found in nature. (WikiPedia) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H12O6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 180.1559 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 180.063388116 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WQZGKKKJIJFFOK-VFUOTHLCSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 492-61-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R,3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | glucoside | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as monosaccharides. These are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Monosaccharides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monosaccharides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Cellobiose + Water <>2 b-D-Glucose Adenosine triphosphate + b-D-Glucose <> ADP + beta-D-Glucose 6-phosphate alpha-D-Glucose <> b-D-Glucose Cellulose + Water <> Cellulose + b-D-Glucose 3-Ketolactose + Water <> 3-Keto-beta-D-galactose + b-D-Glucose b-D-Glucose <> D-glucose Phosphoenolpyruvic acid + b-D-Glucose > Glucose 6-phosphate + Pyruvic acid a β-D glucoside + Water a non glucosylated glucose acceptor + b-D-Glucose Cellobiose-6-phosphate + Water > Glucose 6-phosphate + b-D-Glucose D-Glucose <> b-D-Glucose Water + Melibiose > D-Galactose + b-D-Glucose Maltotriose + D-Maltose <> Maltotetraose + b-D-Glucose Water + alpha-Lactose > D-Galactose + b-D-Glucose GDP-α-D-glucose + Water > Hydrogen ion + b-D-Glucose + Guanosine diphosphate b-D-Glucose + a ubiquinone > Gluconolactone + a ubiquinol b-D-Glucose + Adenosine triphosphate > Hydrogen ion + Glucose 6-phosphate + ADP isoprimeverose + Water b-D-Glucose + D-Xylose Gluconolactone + Hydrogen ion <> b-D-Glucose Trehalose 6-phosphate + Water > Glucose 6-phosphate + b-D-Glucose Trehalose + Water > b-D-Glucose + D-Glucose Trehalose + Water > b-D-Glucose + alpha-D-Glucose alpha-Lactose + Water > beta-D-Galactose + Beta-D-Glucose + b-D-Glucose More...Beta-D-Glucose + Adenosine triphosphate + b-D-Glucose > Hydrogen ion + Adenosine diphosphate + beta-D-Glucose 6-phosphate + ADP Melibiose + Water > Alpha-D-Galactose + Beta-D-Glucose + b-D-Glucose α,α-trehalose + Water > alpha-D-Glucose + Beta-D-Glucose + b-D-Glucose Beta-D-Glucose + HPr - phosphorylated + b-D-Glucose > beta-D-Glucose 6-phosphate + HPr | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Wenck, Helmut; Kinedt, Claudia; Bader, Hans Joachim. Production of b-D-glucose. Praxis der Naturwissenschaften, Chemie (1986), 35(4), 23. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in glucokinase activity
- Specific function:
- Not highly important in Pseudomonas aeruginosa as glucose is transported into the cell by the PTS system already as glucose 6-phosphate
- Gene Name:
- glk
- Locus Tag:
- PA3193
- Molecular weight:
- 34.6 kDa
Reactions
| ATP + D-glucose = ADP + D-glucose 6-phosphate. |
- General function:
- Involved in catalytic activity
- Specific function:
- Provides the cells with the ability to utilize trehalose at high osmolarity by splitting it into glucose molecules that can subsequently be taken up by the phosphotransferase-mediated uptake system
- Gene Name:
- treA
- Locus Tag:
- PA2416
- Molecular weight:
- 61.2 kDa
Reactions
| Alpha,alpha-trehalose + H(2)O = 2 D-glucose. |
- General function:
- Involved in oxidoreductase activity, acting on CH-OH group of donors
- Specific function:
- GDH is probably involved in energy conservation rather than in sugar metabolism
- Gene Name:
- gcd
- Locus Tag:
- PA2290
- Molecular weight:
- 86.2 kDa
Reactions
| D-glucose + ubiquinone = D-glucono-1,5-lactone + ubiquinol. |
- General function:
- Involved in hydrolase activity, hydrolyzing O-glycosyl compounds
- Specific function:
- Hydrolysis of terminal, non-reducing beta-D- glucosyl residues with release of beta-D-glucose
- Gene Name:
- bglX
- Locus Tag:
- PA1726
- Molecular weight:
- 83 kDa
Reactions
| Hydrolysis of terminal, non-reducing beta-D-glucosyl residues with release of beta-D-glucose. |