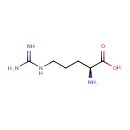
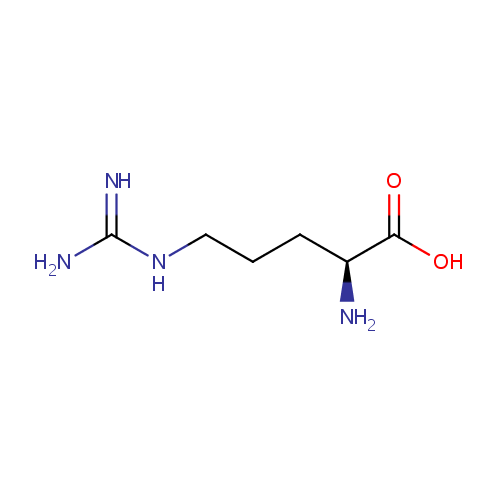
L-Arginine (PAMDB000146)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000146 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | L-Arginine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Arginine is an amino acid that is physiologically active in the L-form. Arginine can be considered to be a basic amino acid as the part of the side chain nearest to the backbone is long, carbon-containing and hydrophobic, whereas the end of the side chain is a complex guanidinium group. With a pKa of 12.48, the guanidinium group is positively charged in neutral, acidic and even most basic environments. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized. This group is able to form multiple H-bonds. The main catabolic arginine pathway of Pseudomonas aeruginosa is the succinyltransferase pathway. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H14N4O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 174.201 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 174.111675712 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ODKSFYDXXFIFQN-BYPYZUCNSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/t4-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 74-79-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-amino-5-carbamimidamidopentanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | L-arginine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | N[C@@H](CCCNC(N)=N)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | L-alpha-amino acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 222 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Water + L-Arginine > ADP + L-Arginine + Hydrogen ion + Phosphate Adenosine triphosphate + Water + L-Arginine > ADP + L-Arginine + Hydrogen ion + Phosphate L-Arginine + Succinyl-CoA <> Coenzyme A + Hydrogen ion + N2-Succinyl-L-arginine L-Arginine + Adenosine triphosphate + tRNA(Arg) + tRNA(Arg) <> Adenosine monophosphate + L-Arginyl-tRNA(Arg) + Pyrophosphate + L-Arginyl-tRNA(Arg) L-Arginine + Hydrogen ion <> Agmatine + Carbon dioxide Argininosuccinic acid <> L-Arginine + Fumaric acid L-Arginine <> Agmatine + Carbon dioxide Succinyl-CoA + L-Arginine <> Coenzyme A + N2-Succinyl-L-arginine Adenosine triphosphate + L-Arginine + tRNA(Arg) <> Adenosine monophosphate + Pyrophosphate + L-Arginyl-tRNA(Arg) -->-->L-arginino-succinate <> L-Arginine + Fumaric acid General-Protein-Substrates L-Arginine + Peptides L-Arginine + Succinyl-CoA + Succinyl-CoA > Coenzyme A + Hydrogen ion + N2-succinyl-L-arginine + N2-succinyl-L-arginine L-Arginine + tRNA(Arg) + Adenosine triphosphate + Hydrogen ion > Pyrophosphate + Adenosine monophosphate + L-arginyl-tRNA(Arg) More...L-Arginine + Adenosine triphosphate + Water > L-Arginine + Adenosine diphosphate + Phosphate + Hydrogen ion + ADP L-Arginine + Adenosine triphosphate + Water > L-Arginine + Adenosine diphosphate + Phosphate + Hydrogen ion + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Meyer, Helmut E.; Swiderek, Kristine; Hoffmann-Posorske, Edeltraut; Korte, Horst; Heilmeyer, Ludwig M. G., Jr. Quantitative determination of phosphoserine by high-performance liquid chromatography as the phenylthiocarbamyl-S-ethylcysteine. Application to | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the binding-protein-dependent transport system for histidine. Probably responsible for energy coupling to the transport system
- Gene Name:
- hisP
- Locus Tag:
- PA2926
- Molecular weight:
- 28.5 kDa
- General function:
- Involved in arginine N-succinyltransferase activity
- Specific function:
- Catalyzes the transfer of succinyl-CoA to arginine to produce N(2)-succinylarginine
- Gene Name:
- astA
- Locus Tag:
- PA0897
- Molecular weight:
- 37.2 kDa
Reactions
| Succinyl-CoA + L-arginine = CoA + N(2)-succinyl-L-arginine. |
- General function:
- Involved in argininosuccinate lyase activity
- Specific function:
- 2-(N(omega)-L-arginino)succinate = fumarate + L-arginine
- Gene Name:
- argH
- Locus Tag:
- PA5263
- Molecular weight:
- 51.6 kDa
Reactions
| 2-(N(omega)-L-arginino)succinate = fumarate + L-arginine. |
- General function:
- Involved in nucleotide binding
- Specific function:
- ATP + L-arginine + tRNA(Arg) = AMP + diphosphate + L-arginyl-tRNA(Arg)
- Gene Name:
- argS
- Locus Tag:
- PA5051
- Molecular weight:
- 65.2 kDa
Reactions
| ATP + L-arginine + tRNA(Arg) = AMP + diphosphate + L-arginyl-tRNA(Arg). |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the biosynthesis of agmatine from arginine
- Gene Name:
- speA
- Locus Tag:
- PA4839
- Molecular weight:
- 70.7 kDa
Reactions
| L-arginine = agmatine + CO(2). |
- General function:
- Involved in transporter activity
- Specific function:
- Part of the binding-protein-dependent transport system for histidine; probably responsible for the translocation of the substrate across the membrane
- Gene Name:
- hisM
- Locus Tag:
- PA2925
- Molecular weight:
- 26.7 kDa
- General function:
- Involved in transporter activity
- Specific function:
- Part of the binding-protein-dependent transport system for histidine; probably responsible for the translocation of the substrate across the membrane
- Gene Name:
- hisQ
- Locus Tag:
- PA2924
- Molecular weight:
- 24.5 kDa
Transporters
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the binding-protein-dependent transport system for histidine. Probably responsible for energy coupling to the transport system
- Gene Name:
- hisP
- Locus Tag:
- PA2926
- Molecular weight:
- 28.5 kDa
- General function:
- Involved in nucleotide binding
- Specific function:
- Probably part of a binding-protein-dependent transport system yecCS for an amino acid. Probably responsible for energy coupling to the transport system
- Gene Name:
- yecC
- Locus Tag:
- PA5152
- Molecular weight:
- 28.4 kDa
- General function:
- Involved in transporter activity
- Specific function:
- Part of the binding-protein-dependent transport system for histidine; probably responsible for the translocation of the substrate across the membrane
- Gene Name:
- hisM
- Locus Tag:
- PA2925
- Molecular weight:
- 26.7 kDa
- General function:
- Involved in transporter activity
- Specific function:
- Part of the binding-protein-dependent transport system for histidine; probably responsible for the translocation of the substrate across the membrane
- Gene Name:
- hisQ
- Locus Tag:
- PA2924
- Molecular weight:
- 24.5 kDa