|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000151 |
|---|
|
Identification |
|---|
| Name: |
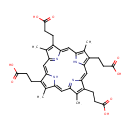
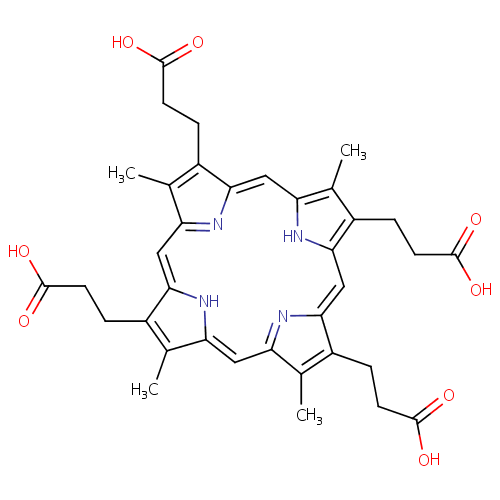
Coproporphyrin III |
|---|
| Description: | Coproporphyrin III is a porphyrin metabolite arising from heme synthesis. Coproporphyrin III is a tetrapyrrole dead-end product from the spontaneous oxidation of the methylene bridges of coproporphynogen, arising from heme synthesis. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3,8,13,17-Tetramethylporphyrin-2,7,12,18-tetrapropanoate
- 3,8,13,17-tetramethylporphyrin-2,7,12,18-tetrapropanoic acid
|
|---|
|
Chemical Formula: |
C36H38N4O8 |
|---|
| Average Molecular Weight: |
654.7089 |
|---|
| Monoisotopic Molecular
Weight: |
654.268964212 |
|---|
| InChI Key: |
JWFCYWSMNRLXLX-UJJXFSCMSA-N |
|---|
| InChI: | InChI=1S/C36H38N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h13-16,37,40H,5-12H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48)/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16- |
|---|
| CAS
number: |
14643-66-4 |
|---|
| IUPAC Name: | 3-[10,14,19-tris(2-carboxyethyl)-5,9,15,20-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8(23),9,11,13,15,17,19-undecaen-4-yl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
coproporphyrin III |
|---|
| SMILES: | CC1=C(CCC(O)=O)/C2=C/C3=N/C(=C\C4=C(C)C(CCC(O)=O)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(C)=C4CCC(O)=O)/C(CCC(O)=O)=C3C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Tetracarboxylic acid or derivatives
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Doss MO: Porphyrinurias and occupational disease. Ann N Y Acad Sci. 1987;514:204-18. Pubmed: 3327428
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Minoda, Taiji; Takada, Toshihiro; Horii, Shinichi. Coproporphyrin III. Jpn. Kokai Tokkyo Koho (1979), 3 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|