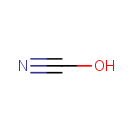Search Results for compounds
Searching compounds for
returned 4373 results.
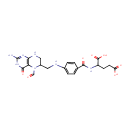
N5-Formyl-H4F (PAMDB000421)
IUPAC:
2-[(4-{[(2-amino-5-formyl-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid
CAS: 58-05-9
Description: N5-Formyl-H4F is the active metabolite of folic acid. Leucovorin is used principally as its calcium salt as an antidote to folic acid antagonists which block the conversion of folic acid to folinic acid.
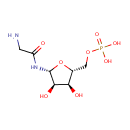
Glycineamideribotide (PAMDB000422)
IUPAC:
{[(2R,3S,4R,5R)-5-(2-aminoacetamido)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid
CAS: 10074-18-7
Description: Glycinamidoribotide conversion to N-formylglycinamide ribonucleotide is the third reaction of the de novo purine biosynthesis, a reaction catalyzed by the enzyme glycinamide ribonucleotide transformylase (EC 2.1.2.2). Glycinamide ribonucleotide (GAR) synthetase catalyzes the conversion of phosphoribosylamine, glycine, and MgATP to glycinamide ribonucleotide.
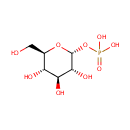
Glucose 1-phosphate (PAMDB000423)
IUPAC:
{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid
CAS: 59-56-3
Description: Glucose 1-phosphate is the direct product of the reaction in which glycogen phosphorylase cleaves off a molecule of glucose from a greater glycogen structure. Glycogen phosphorylase, the product of the glgP Gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Pseudomonas aeruginosa. It cannot travel down many metabolic pathways and must be interconverted by the enzyme phosphoglucomutase in order to become glucose 6-phosphate. In glycogenesis, free glucose 1-phosphate can also react with UTP to form UDP-glucose, by using the enzyme UDP-glucose pyrophosphorylase. Periplasmic acid glucose-1-phosphatase (G-1-Pase) encoded by gene Agp is necessary for the growth of Pseudomonas aeruginosa in a minimal medium containing glucose-1-phosphate (G-1-P) as the sole source of carbon.
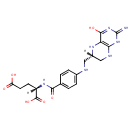
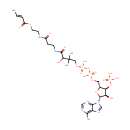
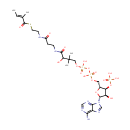
Tetrahydrofolic acid (PAMDB000424)
IUPAC:
(2S)-2-{[4-({[(6S)-4-hydroxy-2-imino-1,2,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)phenyl]formamido}pentanedioic acid
CAS: 135-16-0
Description: Tetrahydrofolate is a soluble coenzyme (vitamin B9) that is synthesized de novo by plants and microorganisms, and absorbed from the diet by animals. It is composed of three distinct parts: a pterin ring, a p-ABA (p-aminobenzoic acid) and a polyglutamate chain with a number of residues varying between 1 and 8. Only the tetra-reduced form of the molecule serves as a coenzyme for C1 transfer reactions. In biological systems, the C1-units exist under various oxidation states and the different tetrahydrofolate derivatives constitute a family of related molecules named indistinctly under the generic term folate. (PMID 16042593)
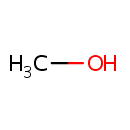
Methanol (PAMDB000425)
IUPAC:
methanol
CAS: 67-56-1
Description: Methanol is the simplest alcohol, and is a light, volatile, colourless, flammable, poisonous liquid with a distinctive odor that is somewhat milder and sweeter than ethanol. It is produced naturally in the anaerobic metabolism of many varieties of bacteria, and is ubiquitous in the environment. As a result, there is a small fraction of methanol vapor in the atmosphere. (Wikipedia).
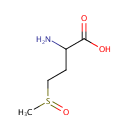
Methionine sulfoxide (PAMDB000428)
IUPAC:
2-amino-4-methanesulfinylbutanoic acid
CAS: 62697-73-8
Description: Methionine sulfoxide is a member of the chemical class known as Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon).Methionine is an amino acid susceptible to being oxidized to methionine sulfoxide (MetSO). The reduction of MetSO to methionine is catalyzed by methionine sulfoxide reductase (MSR), an enzyme present in almost all organisms. (PMID 20969952) Oxidation of methionine to methionine sulfoxide is a major oxidative stress product that reaches levels as high as 60% in cataract while being essentially absent from clear lenses. Methionine oxidation results in loss of protein function that can be reversed through the action of methionine sulfoxide reductase A (MsrA), which is implicated in oxidative stress protection and is an essential regulator of longevity in species ranging from Pseudomonas aeruginosa to mice. (PMID 15199188) A sulfenic acid enzyme intermediate is involved in the catalytic mechanism of peptide methionine sulfoxide reductase from Pseudomonas aeruginosa. (PMID 10964927)
Crotonoyl-CoA (PAMDB000429)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-{[({[(3-{[2-({2-[(2E)-but-2-enoylsulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-3-hydroxy-2,2-dimethylpropoxy)(hydroxy)phosphoryl]oxy}(hydroxy)phosphoryl)oxy]methyl}-4-hydroxyoxolan-3-yl]oxy}phosphonic acid
CAS: 102680-35-3
Description: Crotonoyl-CoA is an important component in several metabolic pathways, notably fatty acid and amino acid metabolism. It is the substrate of a group of enzymes acyl-Coenzyme A oxidases 1, 2, 3 (E.C.: 1.3.3.6) corresponding to palmitoyl, branched chain, and pristanoyl, respectively, in the peroxisomal fatty acid beta-oxidation, producing hydrogen peroxide. It is also a substrate of a group of enzymes called acyl-Coenzyme A dehydrogenase (E.C.:1.3.99-, including 1.3.99.2, 1.3.99.3) in the metabolism of fatty acids. In addition, crotonoyl-CoA is the substrate of enoyl coenzyme A hydratase (E.C.4.2.1.17) during lysine degradation and tryptophan metabolism, benzoate degradation via CoA ligation; in contrast it is the product of this enzyme in the butanoate metabolism. Moreover, it is produced from multiple enzymes in the butanoate metabolism pathway, including 3-Hydroxybutyryl-CoA dehydratase (E.C.:4.2.1.55), glutaconyl-CoA decarboxylase (E.C.: 4.1.1.70), vinylacetyl-CoA delta-isomerase (E.C.: 5.3.3.3), and trans-2-enoyl-CoA reductase (NAD+) (E.C.: 1.3.1.44). In lysine degradation and tryptophan metabolism, crotonoyl CoA is produced by glutaryl-Coenzyme A dehydrogenase (E.C.:1.3.99.7) lysine and tryptophan metabolic pathway.
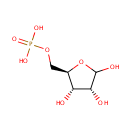
D-Ribose-5-phosphate (PAMDB000431)
IUPAC:
{[(2R,3S,4R)-3,4,5-trihydroxyoxolan-2-yl]methoxy}phosphonic acid
CAS: 4151-19-3
Description: D-ribose-5-phosphate is a member of the chemical class known as Pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. Ribose 5-phosphate is both a product and an intermediate of the pentose phosphate pathway. The last step of the oxidative reactions in the pentose phosphate pathway is the production of ribulose-5-phosphate. Ribulose-5-phosphate can reversibly isomerize to ribose-5-phosphate. Ribulose-5-phosphate can alternatively undergo a series of isomerizations as well as transaldolations and transketolations that result in the production of other pentoses phosphates as well as fructose 6-phosphate and glyceraldehyde-3-phosphate (both intermediates in glycolysis). The enzyme ribose-phosphate diphosphokinase converts ribose-5-phosphate into phosphoribosyl pyrophosphate. (WikiPedia)
Tiglyl-CoA (PAMDB000433)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-2,2-dimethyl-3-({2-[(2-{[(2E)-2-methylbut-2-enoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: Not Available
Description: Tiglyl-CoA is a metabolite in the degradation of isoleucine to propionic acid pathway. It is a substrate of enoyl-CoA hydratase-isomerase [EC:4.2.1.17] and converted to (2S,3S)-3-Hydroxy-2-methylbutanoyl-CoA.
Cyanate (PAMDB000437)
IUPAC:
cyanic acid
CAS: 71000-82-3
Description: The cyanate ion is an anion consisting of one oxygen atom, one carbon atom, and one nitrogen atom, [OCN], in that order. The cyanate ion possesses 1 unit of negative charge, borne mainly by the nitrogen atom. In organic compounds the cyanate group is a functional group.; The cyanate ion is an ambident nucleophile in nucleophilic substitution because it can react to form an alkyl cyanate R-OCN (exception) or an alkyl isocyanate R-NCO (rule). Aryl cyanates (C6H5OCN) can be formed by a reaction of phenol with cyanogen chloride (ClCN) in the presence of a base. The cyanate ion is relatively non-toxic in comparison with cyanides. Use of this fact is made in cyanide decontamination processes where a permanganate oxidation converts toxic cyanide to safer cyanate. Cyanate can be decomposed by the enzyme cyanate lyase (or cyanase), which is found in bacteria and plants. In particular cyanate can be decomposed to carbamate (ammonia) and carbon dioxide. Alternately the same enzyme can be used to synthesize cyanate using carbamate and carbon dioxide.