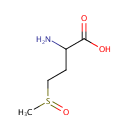
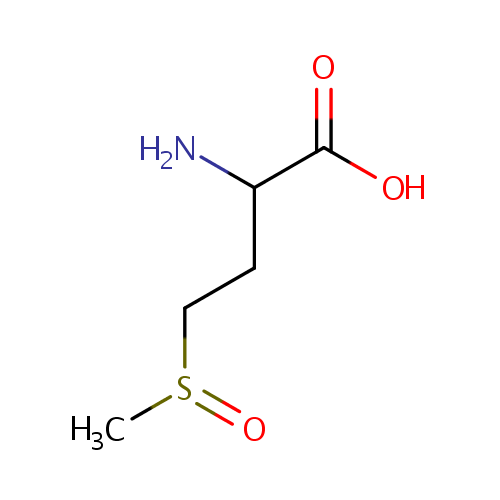
| References: |
- Arias, D. G., Cabeza, M. S., Erben, E. D., Carranza, P. G., Lujan, H. D., Tellez Inon, M. T., Iglesias, A. A., Guerrero, S. A. (2011). "Functional characterization of methionine sulfoxide reductase A from Trypanosoma spp." Free Radic Biol Med 50:37-46. Pubmed: 20969952
- Boudier C, Cadene M, Bieth JG: Inhibition of neutrophil cathepsin G by oxidized mucus proteinase inhibitor. Effect of heparin. Biochemistry. 1999 Jun 29;38(26):8451-7. Pubmed: 10387091
- Kantorow, M., Hawse, J. R., Cowell, T. L., Benhamed, S., Pizarro, G. O., Reddy, V. N., Hejtmancik, J. F. (2004). "Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress." Proc Natl Acad Sci U S A 101:9654-9659. Pubmed: 15199188
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Mashima R, Nakanishi-Ueda T, Yamamoto Y: Simultaneous determination of methionine sulfoxide and methionine in blood plasma using gas chromatography-mass spectrometry. Anal Biochem. 2003 Feb 1;313(1):28-33. Pubmed: 12576054
- O'Donohue TL, Charlton CG, Thoa NB, Helke CJ, Moody TW, Pert A, Williams A, Miller RL, Jacobowitz DM: Release of alpha-melanocyte stimulating hormone into rat and human cerebrospinal fluid in vivo and from rat hypothalamus slices in vitro. Peptides. 1981 Spring;2(1):93-100. Pubmed: 7243627
- Schallreuter KU: Functioning methionine-S-sulfoxide reductases A and B are present in human skin. J Invest Dermatol. 2006 May;126(5):947-9. Pubmed: 16619011
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|