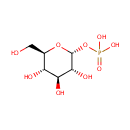
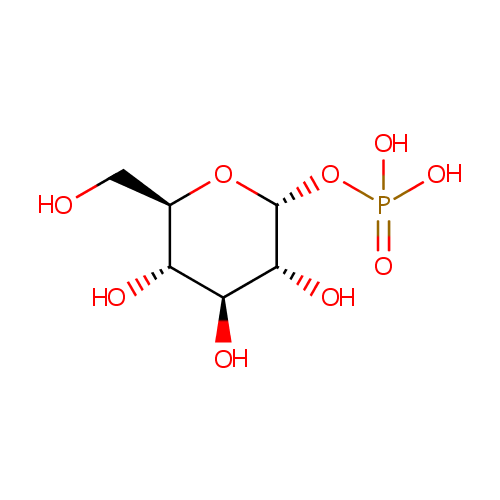
Glucose 1-phosphate (PAMDB000423)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000423 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Glucose 1-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Glucose 1-phosphate is the direct product of the reaction in which glycogen phosphorylase cleaves off a molecule of glucose from a greater glycogen structure. Glycogen phosphorylase, the product of the glgP Gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Pseudomonas aeruginosa. It cannot travel down many metabolic pathways and must be interconverted by the enzyme phosphoglucomutase in order to become glucose 6-phosphate. In glycogenesis, free glucose 1-phosphate can also react with UTP to form UDP-glucose, by using the enzyme UDP-glucose pyrophosphorylase. Periplasmic acid glucose-1-phosphatase (G-1-Pase) encoded by gene Agp is necessary for the growth of Pseudomonas aeruginosa in a minimal medium containing glucose-1-phosphate (G-1-P) as the sole source of carbon. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H13O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 260.1358 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 260.029718526 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | HXXFSFRBOHSIMQ-VFUOTHLCSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H13O9P/c7-1-2-3(8)4(9)5(10)6(14-2)15-16(11,12)13/h2-10H,1H2,(H2,11,12,13)/t2-,3-,4+,5-,6-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 59-56-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | α-D-glucose 1-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC[C@H]1O[C@H](OP(O)(O)=O)[C@H](O)[C@@H](O)[C@@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as monosaccharide phosphates. These are monosaccharides comprising a phosphated group linked to the carbohydrate unit. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Monosaccharides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monosaccharide phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Thymidine 5'-triphosphate + Glucose 1-phosphate + Hydrogen ion <> dTDP-D-Glucose + Pyrophosphate Glucose 1-phosphate <> Glucose 6-phosphate branching glycogen + Phosphate > Glucose 1-phosphate Glycogen + Phosphate > Glucose 1-phosphate Water + UDP-Glucose > Glucose 1-phosphate +2 Hydrogen ion + Uridine 5'-monophosphate Galactose 1-phosphate + UDP-Glucose <> Glucose 1-phosphate + Uridine diphosphategalactose Glucose 1-phosphate + Water > D-Glucose + Phosphate Glucose 1-phosphate + Hydrogen ion + Uridine triphosphate <> Pyrophosphate + UDP-Glucose Maltoheptaose + Phosphate <> Glucose 1-phosphate + Maltohexaose Maltohexaose + Phosphate <> Glucose 1-phosphate + Maltopentaose Maltopentaose + Phosphate <> Glucose 1-phosphate + Maltotetraose Adenosine triphosphate + Glucose 1-phosphate + Hydrogen ion <> ADP-Glucose + Pyrophosphate UDP-Glucose + Water <> Uridine 5'-monophosphate + Glucose 1-phosphate Uridine triphosphate + Glucose 1-phosphate <> Pyrophosphate + UDP-Glucose Sucrose + Phosphate <> D-Fructose + Glucose 1-phosphate Glucose 1-phosphate + Water <> alpha-D-Glucose + Phosphate Adenosine triphosphate + Glucose 1-phosphate <> Pyrophosphate + ADP-Glucose Starch + Phosphate <> 1,4-alpha-D-glucan + Glucose 1-phosphate Thymidine 5'-triphosphate + Glucose 1-phosphate <> Pyrophosphate + dTDP-D-Glucose Glucose 1-phosphate > beta-D-Glucose 1-phosphate Water + Glucose 1-phosphate > Phosphate + D-glucose More...Glycogen + Phosphate <> a limit dextrin + Glucose 1-phosphate Glucose 1-phosphate <> α-D-glucose 6-phosphate Maltotetraose + Phosphate <> Maltotriose + Glucose 1-phosphate a 1,4-α-D-glucan + Phosphate <> a 1,4-α-D-glucan + Glucose 1-phosphate Sucrose + Phosphate <> D-Fructose + Glucose 1-phosphate Phosphate <> Glucose 1-phosphate Glucose 1-phosphate + Uridine triphosphate + Hydrogen ion + Uridine triphosphate > Pyrophosphate + UDP-Glucose Galactose 1-phosphate + Galactose 1-phosphate > Glucose 1-phosphate Galactose 1-phosphate + UDP-Glucose + Galactose 1-phosphate > Uridine diphosphategalactose + Glucose 1-phosphate + Uridine diphosphategalactose Thymidine 5'-triphosphate + Hydrogen ion + Glucose 1-phosphate > Pyrophosphate + dTDP-D-Glucose | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Weinhausel, Andreas; Nidetzky, Bernd; Kysela, Christian; Kulbe, Klaus D. Application of Escherichia coli maltodextrin-phosphorylase for the continuous production of glucose-1-phosphate. Enzyme and Microbial Technology (1995), 17(2), 140-6. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in phosphorylase activity
- Specific function:
- Phosphorylase is an important allosteric enzyme in carbohydrate metabolism. Enzymes from different sources differ in their regulatory mechanisms and in their natural substrates. However, all known phosphorylases share catalytic and structural properties
- Gene Name:
- glgP
- Locus Tag:
- PA2144
- Molecular weight:
- 92 kDa
Reactions
| (1,4-alpha-D-glucosyl)(n) + phosphate = (1,4-alpha-D-glucosyl)(n-1) + alpha-D-glucose 1-phosphate. |
- General function:
- Involved in UTP:glucose-1-phosphate uridylyltransferase activity
- Specific function:
- May play a role in stationary phase survival
- Gene Name:
- galU
- Locus Tag:
- PA2023
- Molecular weight:
- 31.2 kDa
Reactions
| UTP + alpha-D-glucose 1-phosphate = diphosphate + UDP-glucose. |
- General function:
- Involved in nucleoside-triphosphate diphosphatase activity
- Specific function:
- Specific function unknown
- Gene Name:
- mazG
- Locus Tag:
- PA0935
- Molecular weight:
- 31.2 kDa
Reactions
| ATP + H(2)O = AMP + diphosphate. |
- General function:
- Involved in intramolecular transferase activity, phosphotransferases
- Specific function:
- This enzyme participates in both the breakdown and synthesis of glucose
- Gene Name:
- pgm
- Locus Tag:
- PA5131
- Molecular weight:
- 55.6 kDa
Reactions
| Alpha-D-glucose 1-phosphate = alpha-D-glucose 6-phosphate. |
- General function:
- Involved in glucose-1-phosphate thymidylyltransferase activity
- Specific function:
- Catalyzes the formation of dTDP-glucose, from dTTP and glucose 1-phosphate, as well as its pyrophosphorolysis
- Gene Name:
- rmlA1
- Locus Tag:
- PA5163
- Molecular weight:
- 32.5 kDa
Reactions
| dTTP + alpha-D-glucose 1-phosphate = diphosphate + dTDP-glucose. |