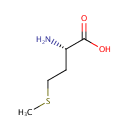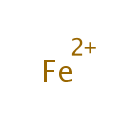| Identification |
| Name: |
Lipoyl synthase |
| Synonyms: |
- Lip-syn
- LS
- Lipoate synthase
- Lipoic acid synthase
- Sulfur insertion protein lipA
|
| Gene Name: |
lipA |
| Enzyme Class: |
|
| Biological Properties |
| General Function: |
Involved in catalytic activity |
| Specific Function: |
Catalyzes the radical-mediated insertion of two sulfur atoms into the C-6 and C-8 positions of the octanoyl moiety bound to the lipoyl domains of lipoate-dependent enzymes, thereby converting the octanoylated domains into lipoylated derivatives. Free octanoate is not a substrate for lipA |
| Cellular Location: |
Cytoplasm |
| KEGG Pathways: |
|
| KEGG Reactions: |
|
Protein N6-(octanoyl)lysine | + | 2 Sulfur donor | + | 2  | + | Protein N6-(octanoyl)lysine | ↔ | Protein N6-(lipoyl)lysine | + | 2 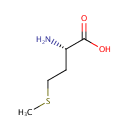 | + | 2 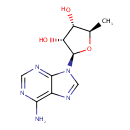 | + | Protein N6-(lipoyl)lysine |
| | |
Octanoyl-[acp] | + | 2 Sulfur donor | + | 2  | ↔ | Lipoyl-[acp] | + | 2 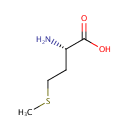 | + | 2  |
| |
|
| SMPDB Reactions: |
|
| PseudoCyc/BioCyc Reactions: |
|
[4Fe-4S] iron-sulfur cluster | + | 2  | + |  | + | 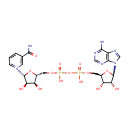 | + | octanoate (protein bound) | → | [2Fe-2S] iron-sulfur cluster | + | 2  | + | 2 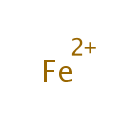 | + | lipoate (protein bound) | + | 2 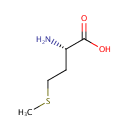 | + | 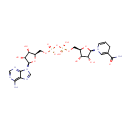 |
| | |
Protein N6-(octanoyl)lysine | + | 2 Sulfur donor | + | 2  | + | Protein N6-(octanoyl)lysine | ↔ | Protein N6-(lipoyl)lysine | + | 2 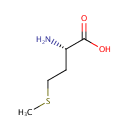 | + | 2  | + | Protein N6-(lipoyl)lysine |
| | |
Octanoyl-[acp] | + | 2 Sulfur donor | + | 2  | ↔ | Lipoyl-[acp] | + | 2 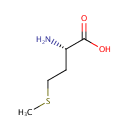 | + | 2  |
| | |
Octanoyl-[acyl-carrier protein] | + | 2 a sulfur donor | + | 2 S-adenosyl-L-methionine | → | Lipoyl-ACP | + | 2 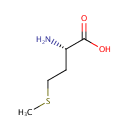 | + |  |
| | |
Protein N6-(octanoyl)lysine | + | 2 a sulfur donor | + | 2 S-adenosyl-L-methionine | → | Protein N6-(lipoyl)lysine | + | 2 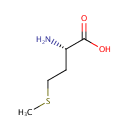 | + | 2  |
| |
|
| Complex Reactions: |
|
[4Fe-4S] iron-sulfur cluster | + | 2  | + |  | + |  | + | octanoate (protein bound) | → | [2Fe-2S] iron-sulfur cluster | + | 2  | + | 2 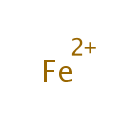 | + | lipoate (protein bound) | + | 2 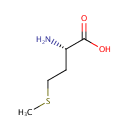 | + |  |
| |
|
| Transports: |
Not Available |
| Metabolites: |
|
| GO Classification: |
| Function |
|---|
| 4 iron, 4 sulfur cluster binding | | binding | | catalytic activity | | iron-sulfur cluster binding | | lipoate synthase activity | | metal cluster binding | | sulfurtransferase activity | | transferase activity | | transferase activity, transferring sulfur-containing groups | | Process |
|---|
| cellular metabolic process | | coenzyme metabolic process | | cofactor metabolic process | | lipoate biosynthetic process | | lipoic acid biosynthetic process | | lipoic acid metabolic process | | metabolic process |
|
| Gene Properties |
| Locus tag: |
PA2862 |
| Strand: |
+ |
| Entrez Gene ID: |
882662 |
| Accession: |
NP_251552.1 |
| GI: |
15598058 |
| Sequence start: |
3214282 |
| Sequence End: |
3215217 |
| Sequence Length: |
935 |
| Gene Sequence: |
>PA2862
ATGAAGAAGAAGTCTCTGCTCCCCCTCGGCCTGGCCATCGGTCTCGCCTCTCTCGCTGCCAGCCCTCTGATCCAGGCCAGCACCTACACCCAGACCAAATACCCCATCGTGCTGGCCCACGGCATGCTCGGCTTCGACAACATCCTCGGGGTCGACTACTGGTTCGGCATTCCCAGCGCCTTGCGCCGTGACGGTGCCCAGGTCTACGTCACCGAAGTCAGCCAGTTGGACACCTCGGAAGTCCGCGGCGAGCAGTTGCTGCAACAGGTGGAGGAAATCGTCGCCCTCAGCGGCCAGCCCAAGGTCAACCTGATCGGCCACAGCCACGGCGGGCCGACCATCCGCTACGTCGCCGCCGTACGTCCCGACCTGATCGCTTCCGCCACCAGCGTCGGCGCCCCGCACAAGGGTTCGGACACCGCCGACTTCCTGCGCCAGATCCCACCGGGTTCGGCCGGCGAGGCAGTCCTCTCCGGGCTGGTCAACAGCCTCGGCGCGCTGATCAGCTTCCTTTCCAGCGGCAGCACCGGTACGCAGAATTCACTGGGCTCGCTGGAGTCGCTGAACAGCGAGGGTGCCGCGCGCTTCAACGCCAAGTACCCGCAGGGCATCCCCACCTCGGCCTGCGGCGAAGGCGCCTACAAGGTCAACGGCGTGAGCTATTACTCCTGGAGCGGTTCCTCGCCGCTGACCAACTTCCTCGATCCGAGCGACGCCTTCCTCGGCGCCTCGTCGCTGACCTTCAAGAACGGCACCGCCAACGACGGCCTGGTCGGCACCTGCAGTTCGCACCTGGGCATGGTGATCCGCGACAACTACCGGATGAACCACCTGGACGAGGTGAACCAGGTCTTCGGCCTCACCAGCCTGTTCGAGACCAGCCCGGTCAGCGTCTACCGCCAGCACGCCAACCGCCTGAAGAACGCCAGCCTGTAG |
| Protein Properties |
| Protein Residues: |
311 |
| Protein Molecular Weight: |
32.7 kDa |
| Protein Theoretical pI: |
6.88 |
| Hydropathicity (GRAVY score): |
-0.008 |
| Charge at pH 7 (predicted): |
-0.39 |
| Protein Sequence: |
>PA2862
MKKKSLLPLGLAIGLASLAASPLIQASTYTQTKYPIVLAHGMLGFDNILGVDYWFGIPSALRRDGAQVYVTEVSQLDTSEVRGEQLLQQVEEIVALSGQPKVNLIGHSHGGPTIRYVAAVRPDLIASATSVGAPHKGSDTADFLRQIPPGSAGEAVLSGLVNSLGALISFLSSGSTGTQNSLGSLESLNSEGAARFNAKYPQGIPTSACGEGAYKVNGVSYYSWSGSSPLTNFLDPSDAFLGASSLTFKNGTANDGLVGTCSSHLGMVIRDNYRMNHLDEVNQVFGLTSLFETSPVSVYRQHANRLKNASL |
| References |
| External Links: |
|
| General Reference: |
PaperBLAST - Find papers about PA2862 and its homologs
|