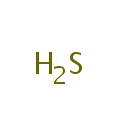Hydrogen sulfide (PAMDB000486)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000486 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Hydrogen sulfide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Hydrogen sulfide is a highly toxic and flammable gas. Because it is heavier than air it tends to accumulate at the bottom of poorly ventilated spaces. Although very pungent at first, it quickly deadens the sense of smell, so potential victims may be unaware of its presence until it is too late. H2S arises from virtually anywhere where elemental sulfur comes into contact with organic material, especially at high temperatures. Hydrogen sulfide is a covalent hydride chemically related to water (H2O) since oxygen and sulfur occur in the same periodic table group. It often results when bacteria break down organic matter in the absence of oxygen, such as in swamps, and sewers (alongside the process of anaerobic digestion). It also occurs in volcanic gases, natural gas and some well waters. It is also important to note that Hydrogen sulfide is a central participant in the sulfur cycle, the biogeochemical cycle of sulfur on earth. As mentioned above, sulfur-reducing and sulfate-reducing bacteria derive energy from oxidizing hydrogen or organic molecules in the absence of oxygen by reducing sulfur or sulfate to hydrogen sulfide. Other bacteria liberate hydrogen sulfide from sulfur-containing amino acids. Several groups of bacteria can use hydrogen sulfide as fuel, oxidizing it to elemental sulfur or to sulfate by using oxygen or nitrate as oxidant. The purple sulfur bacteria and the green sulfur bacteria use hydrogen sulfide as electron donor in photosynthesis, thereby producing elemental sulfur. (In fact, this mode of photosynthesis is older than the mode of cyanobacteria, algae and plants which uses water as electron donor and liberates oxygen). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | H2S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 34.081 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 33.987720754 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | RWSOTUBLDIXVET-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/H2S/h1H2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 7783-06-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | hydrogen sulfide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | hydrogen sulfide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as homogeneous other non-metal compounds. These are inorganic non-metallic compounds in which the largest atom belongs to the class of 'other nonmetals'. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Homogeneous other non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Homogeneous other non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | -85.49 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | O-Acetylserine + Hydrogen sulfide <> Acetic acid + L-Cysteine + Hydrogen ion 5 Hydrogen ion + 3 NADPH + Sulfite <>3 Water + Hydrogen sulfide +3 NADP L-Cysteine + Water > Hydrogen sulfide + Ammonium + Pyruvic acid D-Cysteine + Water > Hydrogen sulfide + Ammonium + Pyruvic acid L-Cysteine + Water <> Hydrogen sulfide + Pyruvic acid + Ammonia O-Acetylserine + Hydrogen sulfide <> L-Cysteine + Acetic acid O-Succinyl-L-homoserine + Hydrogen sulfide <> L-Homocysteine + Succinic acid D-Cysteine + Water <> Hydrogen sulfide + Ammonia + Pyruvic acid Hydrogen ion + 3-Mercaptopyruvic acid > Pyruvic acid + Hydrogen sulfide D-Cysteine + Water <> Pyruvic acid + Hydrogen sulfide + Ammonia + Hydrogen ion L-Cysteine + Water > Pyruvic acid + Ammonia + Hydrogen sulfide + Hydrogen ion Hydrogen sulfide + 3 NADP + 3 Water > Sulfite +3 NADPH L-Cysteine > Hydrogen ion + Hydrogen sulfide + 2-Aminoacrylic acid 3 NADPH + 5 Hydrogen ion + Sulfite + 3 NADPH + Sulfite > Hydrogen sulfide +3 Water +3 NADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||