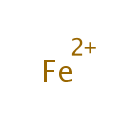Iron (PAMDB000172)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000172 | |||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Iron | |||||||||||||||||||||||||||||||||||||||||||||
| Description: | Iron is a chemical element with the symbol Fe and atomic number 26. Iron makes up 5% of the Earth's crust and is second in abundance to aluminium among the metals and fourth in abundance among the elements. Iron (as Fe2+, ferrous ion) is a necessary trace element used by all known living organisms. Iron-containing enzymes, usually containing heme prosthetic groups, participate in catalysis of oxidation reactions in biology, and in transport of a number of soluble gases. Its chief functions are in the transport of oxygen to tissue (hemoglobin) and in cellular oxidation mechanisms. Inorganic iron involved in redox reactions is also found in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. A class of non-heme iron proteins is responsible for a wide range of functions such as ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis) and purple acid phosphatase (hydrolysis of phosphate esters). When the body is fighting a bacterial infection, the body sequesters iron inside of cells (mostly stored in the storage molecule ferritin) so that it cannot be used by bacteria. Iron may promote both growth of Pseudomonas aeruginosa. | |||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | Fe | |||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 55.845 | |||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 55.934942133 | |||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | CWYNVVGOOAEACU-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/Fe/q+2 | |||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 7439-89-6 | |||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | ??-iron(2+) ion | |||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | ??-iron(2+) ion | |||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [Fe++] | |||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. | |||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous metal compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Class | Homogeneous transition metal compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Homogeneous transition metal compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 2 | |||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 1538 °C | |||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + FADH2 + 2 Iron + Water + SufBCD scaffold complex + 2 SufSE with bound sulfur > ADP + FAD +7 Hydrogen ion + Phosphate + SufBCD with bound [2Fe-2S] cluster +2 SufSE sulfur acceptor complex Adenosine triphosphate + FADH2 + 2 Iron + Water + SufBCD with bound [2Fe-2S] cluster + 2 SufSE with bound sulfur > ADP + FAD +7 Hydrogen ion + Phosphate + SufBCD with two bound [2Fe-2S] clusters +2 SufSE sulfur acceptor complex FADH2 + 2 Iron + 2 IscS with bound sulfur + IscU scaffold protein > FAD +6 Hydrogen ion +2 IscS sulfur acceptor protein + IscU with bound [2Fe-2S] cluster FADH2 + 2 Iron + 2 IscS with bound sulfur + IscU with bound [2Fe-2S] cluster > FAD +6 Hydrogen ion +2 IscS sulfur acceptor protein + IscU with two bound [2Fe-2S] clusters 4 Iron + 4 Hydrogen ion + Oxygen >4 Fe3+ +2 Water [4Fe-4S] iron-sulfur cluster + 2 S-Adenosylmethionine + Hydrogen ion + NAD + octanoate (protein bound) > [2Fe-2S] iron-sulfur cluster +2 5'-Deoxyadenosine +2 Iron + lipoate (protein bound) +2 L-Methionine + NADH Iron + Sirohydrochlorin >3 Hydrogen ion + Siroheme Adenosine triphosphate + Water + Iron > ADP + Iron + Hydrogen ion + Phosphate Adenosine triphosphate + Water + Iron > ADP + Iron + Hydrogen ion + Phosphate FADH2 + 2 Fe3+ > FAD +2 Iron +2 Hydrogen ion [3Fe-4S] damaged iron-sulfur cluster + Iron > [4Fe-4S] iron-sulfur cluster FADH2 + 2 Ferroxamine > FAD +2 Iron +2 ferroxamine minus Fe(3) +2 Hydrogen ion 2 Ferroxamine + FMNH >2 Iron +2 ferroxamine minus Fe(3) + Flavin Mononucleotide +2 Hydrogen ion 2 Ferroxamine + Reduced riboflavin >2 Iron +2 ferroxamine minus Fe(3) +2 Hydrogen ion + Riboflavin Hydrogen ion + Hydrogen peroxide + Iron > hydroxyl radical + OH<SUP>-</SUP> + Fe<SUP>3+</SUP> Oxygen + Iron > Superoxide anion + Fe<SUP>3+</SUP> Iron + Protoporphyrin IX > Heme + Hydrogen ion Iron + Hydrogen ion + Oxygen > Fe<SUP>3+</SUP> + Water Iron + a siderophore + NADP < an Fe(III)-siderophore + NADPH + Hydrogen ion Iron + (2,3-dihydroxybenzoylserine)<sub>3</sub> + NADP < ferric 2,3-dihydroxybenzoylserine + NADPH + Hydrogen ion 2 Iron + Hydrogen peroxide + 2 Hydrogen ion >2 Fe3+ +2 Water More...2 Iron + 2 an apo-siderophore + NADP + Hydrogen ion >2 an Fe(III)-siderophore + NADPH Protoporphyrin IX + Iron >2 Hydrogen ion + ferroheme b | |||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | |||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in metabolic process
- Specific function:
- Catalyzes the removal of elemental sulfur and selenium atoms from cysteine and selenocysteine to produce alanine. Functions as a sulfur delivery protein for NAD, biotin and Fe-S cluster synthesis. Transfers sulfur on 'Cys-456' of thiI in a transpersulfidation reaction. Transfers sulfur on 'Cys-19' of tusA in a transpersulfidation reaction. Functions also as a selenium delivery protein in the pathway for the biosynthesis of selenophosphate
- Gene Name:
- iscS
- Locus Tag:
- PA3814
- Molecular weight:
- 44.7 kDa
Reactions
| L-cysteine + acceptor = L-alanine + S-sulfanyl-acceptor. |
- General function:
- Involved in methyltransferase activity
- Specific function:
- Multifunctional enzyme that catalyzes the SAM-dependent methylation of uroporphyrinogen III at position C-2 and C-7 to form precorrin-2 and then position C-12 or C-18 to form trimethylpyrrocorphin 2. It also catalyzes the conversion of precorrin-2 into siroheme. This reaction consists of the NAD- dependent oxidation of precorrin-2 into sirohydrochlorin and its subsequent ferrochelation into siroheme
- Gene Name:
- cysG
- Locus Tag:
- PA2611
- Molecular weight:
- 50.4 kDa
Reactions
| S-adenosyl-L-methionine + uroporphyrinogen III = S-adenosyl-L-homocysteine + precorrin-1. |
| S-adenosyl-L-methionine + precorrin-1 = S-adenosyl-L-homocysteine + precorrin-2. |
| Precorrin-2 + NAD(+) = sirohydrochlorin + NADH. |
| Siroheme + 2 H(+) = sirohydrochlorin + Fe(2+). |
- General function:
- Involved in ferrochelatase activity
- Specific function:
- Catalyzes the ferrous insertion into protoporphyrin IX
- Gene Name:
- hemH
- Locus Tag:
- PA4655
- Molecular weight:
- 38.6 kDa
Reactions
| Protoheme + 2 H(+) = protoporphyrin + Fe(2+). |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the radical-mediated insertion of two sulfur atoms into the C-6 and C-8 positions of the octanoyl moiety bound to the lipoyl domains of lipoate-dependent enzymes, thereby converting the octanoylated domains into lipoylated derivatives. Free octanoate is not a substrate for lipA
- Gene Name:
- lipA
- Locus Tag:
- PA2862
- Molecular weight:
- 32.7 kDa
Reactions
| Protein N(6)-(octanoyl)lysine + 2 sulfur + 2 S-adenosyl-L-methionine = protein N(6)-(lipoyl)lysine + 2 L-methionine + 2 5'-deoxyadenosine. |
- General function:
- Involved in GTP binding
- Specific function:
- GTP-driven Fe(2+) uptake system
- Gene Name:
- feoB
- Locus Tag:
- PA4358
- Molecular weight:
- 82.5 kDa
- General function:
- Involved in iron ion binding
- Specific function:
- May be involved in the formation or repair of [Fe-S] clusters present in iron-sulfur proteins (Potential)
- Gene Name:
- nifU
- Locus Tag:
- PA3813
- Molecular weight:
- 13.8 kDa
- General function:
- Not Available
- Specific function:
- Not Available
- Gene Name:
- ftnA
- Locus Tag:
- PA4235
- Molecular weight:
- 17.9 kDa
- General function:
- Inorganic ion transport and metabolism
- Specific function:
- May perform analogous functions in iron detoxification and storage to that of animal ferritins
- Gene Name:
- bfr
- Locus Tag:
- PA3531
- Molecular weight:
- 18.6 kDa
Reactions
| 4 Fe(2+) + 4 H(+) + O(2) = 4 Fe(3+) + 2 H(2)O. |
Transporters
- General function:
- Involved in GTP binding
- Specific function:
- GTP-driven Fe(2+) uptake system
- Gene Name:
- feoB
- Locus Tag:
- PA4358
- Molecular weight:
- 82.5 kDa