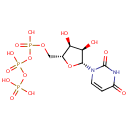
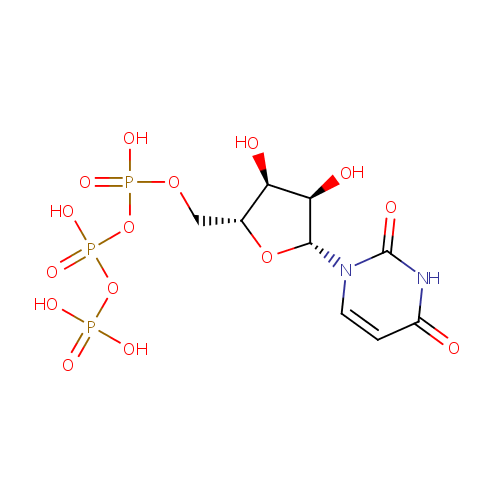
Uridine triphosphate (PAMDB000117)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000117 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Uridine triphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Uridine 5'-(tetrahydrogen triphosphate). A uracil nucleotide containing three phosphate groups esterified to the sugar moiety. Uridine triphosphate has the role of a source of energy or an activator of substrates in metabolic reactions, like that of adenosine triphosphate, but more specific. When Uridine triphosphate activates a substrate, UDP-substrate is usually formed and inorganic phosphate is released. (Wikipedia) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H15N2O15P3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 484.1411 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 483.968527356 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PGAVKCOVUIYSFO-XVFCMESISA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H15N2O15P3/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(24-8)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,21,22)(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 63-39-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | ({[({[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | uridine 5'-triphosphoric acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(O)(=O)OP(O)(=O)OP(O)(O)=O)N1C=CC(=O)NC1=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine ribonucleoside triphosphates. These are pyrimidine ribobucleotides with triphosphate group linked to the ribose moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine ribonucleoside triphosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 Flavodoxin reduced + 2 Hydrogen ion + Uridine triphosphate > Deoxyuridine triphosphate +2 flavodoxin semi oxidized + Water Adenosine triphosphate + Uridine 5'-diphosphate <> ADP + Uridine triphosphate Glucose 1-phosphate + Hydrogen ion + Uridine triphosphate <> Pyrophosphate + UDP-Glucose Adenosine triphosphate + L-Glutamine + Water + Uridine triphosphate > ADP + Cytidine triphosphate + L-Glutamate +2 Hydrogen ion + Phosphate Water + Uridine triphosphate > Hydrogen ion + Pyrophosphate + Uridine 5'-monophosphate N-Acetyl-glucosamine 1-phosphate + Hydrogen ion + Uridine triphosphate > Pyrophosphate + Uridine diphosphate-N-acetylglucosamine Uridine triphosphate + Glucose 1-phosphate <> Pyrophosphate + UDP-Glucose Uridine triphosphate + Glucosamine-1P <> Pyrophosphate + Uridine diphosphate-N-acetylglucosamine Uridine triphosphate + RNA <> Pyrophosphate + RNA Uridine triphosphate + Cytidine <> Uridine 5'-diphosphate + Cytidine monophosphate Cytidine triphosphate + Water <> Uridine triphosphate + Ammonia Adenosine triphosphate + Uridine triphosphate + Ammonia <> ADP + Phosphate + Cytidine triphosphate Adenosine triphosphate + Uridine triphosphate + L-Glutamine + Water <> ADP + Phosphate + Cytidine triphosphate + L-Glutamate Uridine triphosphate + Water <> Uridine 5'-monophosphate + Pyrophosphate Uridine triphosphate + Uridine <> Uridine 5'-diphosphate + Uridine 5'-monophosphate Deoxyuridine triphosphate + Thioredoxin disulfide + Water <> Uridine triphosphate + Thioredoxin Uridine triphosphate + D-Tagatose 6-phosphate <> Uridine 5'-diphosphate + D-Tagatose 1,6-bisphosphate Hydrogen ion + <i>N</i>-acetyl-α-D-glucosamine 1-phosphate + Uridine triphosphate > Uridine diphosphate-N-acetylglucosamine + Pyrophosphate Uridine triphosphate + [protein-PII] > Pyrophosphate + uridylyl-[protein-PII] More...Adenosine triphosphate + Uridine triphosphate + Ammonia > ADP + Inorganic phosphate + Cytidine triphosphate Adenosine triphosphate + Uridine triphosphate + L-Glutamine + Water + Ammonia <> ADP + Phosphate + Cytidine triphosphate + L-Glutamate Glucose 1-phosphate + Uridine triphosphate + Hydrogen ion + Uridine triphosphate > Pyrophosphate + UDP-Glucose β-D-glucose 1-phosphate + Uridine triphosphate + Hydrogen ion + Uridine triphosphate > UDP-Glucose + Pyrophosphate Alpha-D-glucose 1-phosphate + Uridine triphosphate + Hydrogen ion + Uridine triphosphate > Pyrophosphate + UDP-Glucose N-Acetyl-glucosamine 1-phosphate + Uridine triphosphate + Hydrogen ion + N-Acetyl-glucosamine 1-phosphate + Uridine triphosphate > Uridine diphosphate-N-acetylglucosamine + Pyrophosphate Uridine 5'-diphosphate + Adenosine triphosphate + Uridine 5'-diphosphate > Uridine triphosphate + Adenosine diphosphate + Uridine triphosphate + ADP Uridine triphosphate + a reduced flavodoxin + Uridine triphosphate > Water + an oxidized flavodoxin + Deoxyuridine triphosphate Uridine triphosphate + L-Glutamine + Water + Adenosine triphosphate + Uridine triphosphate > Adenosine diphosphate + Hydrogen ion + Phosphate + L-Glutamic acid + Cytidine triphosphate + ADP + L-Glutamate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Kenner, G. W.; Todd, A. R.; Webb, R. F.; Weymouth, F. J. Nucleotides. XXVIII. Synthesis of uridine 5'-triphosphate. Journal of the Chemical Society (1954), 46-52 2288-93. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in nucleoside diphosphate kinase activity
- Specific function:
- Major role in the synthesis of nucleoside triphosphates other than ATP. The ATP gamma phosphate is transferred to the NDP beta phosphate via a ping-pong mechanism, using a phosphorylated active-site intermediate
- Gene Name:
- ndk
- Locus Tag:
- PA3807
- Molecular weight:
- 15.6 kDa
Reactions
| ATP + nucleoside diphosphate = ADP + nucleoside triphosphate. |
- General function:
- Involved in CTP synthase activity
- Specific function:
- Catalyzes the ATP-dependent amination of UTP to CTP with either L-glutamine or ammonia as the source of nitrogen
- Gene Name:
- pyrG
- Locus Tag:
- PA3637
- Molecular weight:
- 59.6 kDa
Reactions
| ATP + UTP + NH(3) = ADP + phosphate + CTP. |
- General function:
- Involved in DNA-directed RNA polymerase activity
- Specific function:
- DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates. This subunit plays an important role in subunit assembly since its dimerization is the first step in the sequential assembly of subunits to form the holoenzyme
- Gene Name:
- rpoA
- Locus Tag:
- PA4238
- Molecular weight:
- 36.6 kDa
Reactions
| Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1). |
- General function:
- Involved in DNA-directed RNA polymerase activity
- Specific function:
- Promotes RNA polymerase assembly. Latches the N- and C- terminal regions of the beta' subunit thereby facilitating its interaction with the beta and alpha subunits
- Gene Name:
- rpoZ
- Locus Tag:
- PA5337
- Molecular weight:
- 9.8 kDa
Reactions
| Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1). |
- General function:
- Involved in DNA binding
- Specific function:
- DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates
- Gene Name:
- rpoC
- Locus Tag:
- PA4269
- Molecular weight:
- 154.4 kDa
Reactions
| Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1). |
- General function:
- Involved in DNA binding
- Specific function:
- DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates
- Gene Name:
- rpoB
- Locus Tag:
- PA4270
- Molecular weight:
- 150.8 kDa
Reactions
| Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1). |
- General function:
- Involved in [formate-C-acetyltransferase]-activating enzyme activity
- Specific function:
- Activation of anaerobic ribonucleoside-triphosphate reductase under anaerobic conditions by generation of an organic free radical, using S-adenosylmethionine and reduced flavodoxin as cosubstrates to produce 5'-deoxy-adenosine
- Gene Name:
- nrdG
- Locus Tag:
- PA1919
- Molecular weight:
- 25.7 kDa
- General function:
- Involved in magnesium ion binding
- Specific function:
- Catalyzes the last two sequential reactions in the de novo biosynthetic pathway for UDP-GlcNAc. Responsible for the acetylation of Glc-N-1-P to give GlcNAc-1-P and for the uridyl transfer from UTP to GlcNAc-1-P which produces UDP-GlcNAc
- Gene Name:
- glmU
- Locus Tag:
- PA5552
- Molecular weight:
- 48.9 kDa
Reactions
| Acetyl-CoA + alpha-D-glucosamine 1-phosphate = CoA + N-acetyl-alpha-D-glucosamine 1-phosphate. |
| UTP + N-acetyl-alpha-D-glucosamine 1-phosphate = diphosphate + UDP-N-acetyl-D-glucosamine. |
- General function:
- Involved in UTP:glucose-1-phosphate uridylyltransferase activity
- Specific function:
- May play a role in stationary phase survival
- Gene Name:
- galU
- Locus Tag:
- PA2023
- Molecular weight:
- 31.2 kDa
Reactions
| UTP + alpha-D-glucose 1-phosphate = diphosphate + UDP-glucose. |
- General function:
- Involved in nucleoside-triphosphate diphosphatase activity
- Specific function:
- Specific function unknown
- Gene Name:
- mazG
- Locus Tag:
- PA0935
- Molecular weight:
- 31.2 kDa
Reactions
| ATP + H(2)O = AMP + diphosphate. |
- General function:
- Involved in amino acid binding
- Specific function:
- Modifies, by uridylylation or deuridylylation the PII (glnB) regulatory protein
- Gene Name:
- glnD
- Locus Tag:
- PA3658
- Molecular weight:
- 103.4 kDa
Reactions
| UTP + [protein-PII] = diphosphate + uridylyl-[protein-PII]. |
- General function:
- Involved in catalytic activity
- Specific function:
- 2'-deoxyribonucleoside triphosphate + thioredoxin disulfide + H(2)O = ribonucleoside triphosphate + thioredoxin
- Gene Name:
- nrdD
- Locus Tag:
- PA1920
- Molecular weight:
- 76.1 kDa
Reactions
| 2'-deoxyribonucleoside triphosphate + thioredoxin disulfide + H(2)O = ribonucleoside triphosphate + thioredoxin. |
- General function:
- Involved in hydrolase activity
- Specific function:
- Hydrolyzes O6 atom-containing purine bases deoxyinosine triphosphate (dITP) and xanthosine triphosphate (XTP) as well as 2'-deoxy-N-6-hydroxylaminopurine triposphate (dHAPTP) to nucleotide monophosphate and pyrophosphate. Probably excludes non- standard purines from DNA precursor pool, preventing thus incorporation into DNA and avoiding chromosomal lesions
- Gene Name:
- rdgB
- Locus Tag:
- PA0387
- Molecular weight:
- 21.2 kDa
Reactions
| A nucleoside triphosphate + H(2)O = a nucleotide + diphosphate. |
- General function:
- Involved in ATP binding
- Specific function:
- Catalyzes the reversible transfer of the terminal phosphate group between ATP and AMP. This small ubiquitous enzyme involved in the energy metabolism and nucleotide synthesis, is essential for maintenance and cell growth
- Gene Name:
- adk
- Locus Tag:
- PA3686
- Molecular weight:
- 23.1 kDa
Reactions
| ATP + AMP = 2 ADP. |