Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 4221 - 4230 of
4373 in total
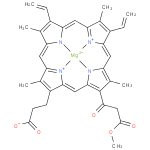
131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester (PAMDB120520)
IUPAC:
{3- [18-
[18- (3-
(3- methoxy-
methoxy- 3-
3- oxopropanoyl)-
oxopropanoyl)- 3,7,12,17-
3,7,12,17- tetramethyl-
tetramethyl- 8,13-
8,13- divinylporphyrin-
divinylporphyrin- 2-
2- yl-
yl- κ4N21,N22,N23,N24]propanoato(3−)}magnesate(1−)
κ4N21,N22,N23,N24]propanoato(3−)}magnesate(1−)
CAS: Not Available
Description: The conjugate base of magnesium 131-oxoprotoporphyrin 13-monomethyl ester, formed by deprotonation of the carboxyethyl group at C-17. It is the principal species at pH 7.3.
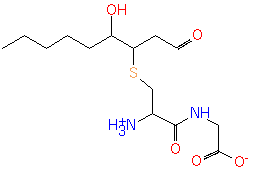
4-hydroxy-2-nonenal-[Cys-Gly] conjugate (PAMDB120521)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
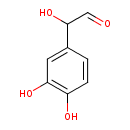
3,4-dihydroxyphenylglycolaldehyde (PAMDB120522)
IUPAC:
2-(3,4-dihydroxyphenyl)-2-hydroxyacetaldehyde
CAS: 13023-73-9
Description: An aldehyde consisting of phenylacetaldehyde having three hydroxy substituents located at the α-, 3- and 4-positions
glucosyl-(heptosyl)2-Kdo2-lipid A (PAMDB120523)
IUPAC:
Not Available
CAS: Not Available
Description: A lipid A oxoanion obtained via deprotonation of the carboxy and phosphate OH groups of glucosyl-(heptosyl)2-(KDO)2-lipid A; major species at pH 7.3.
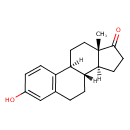
estrone (PAMDB120524)
IUPAC:
(1S,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7.0.0?,??0??,???heptadeca-2(7),3,5-trien-14-one
CAS: 53-16-7
Description: The main Estrogens estrogens in humans are estrone, 17β-estradiol and estriol, which differ in the number of hydroxyl groups (one for estrone, two for 17β-estradiol and three for estriol). 17β-estradiol is about 10 times as potent as estrone and about 80 times as potent as estriol in its estrogenic effect.
17β-estradiol is the predominant estrogen in non-pregenant women during their reproductive years in terms of serum levels. estrone Estrone is the predominant circulating estrogen in women during menopause and estriol is the predominant circulating estrogen during pregnancy.
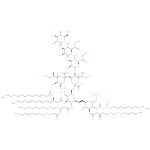
hexadecanedioyl-CoA (PAMDB120525)
IUPAC:
3'- phosphonatoadenosine 5'-
phosphonatoadenosine 5'- {3-
{3- [(3R)-
[(3R)- 4-
4- ({3-
({3- [(2-
[(2- {[15-
{[15- carboxylatopentadecanoyl]sulfanyl}ethyl)amino]-
carboxylatopentadecanoyl]sulfanyl}ethyl)amino]- 3-
3- oxopropyl}amino)-
oxopropyl}amino)- 3-
3- hydroxy-
hydroxy- 2,2-
2,2- dimethyl-
dimethyl- 4-
4- oxobutyl] diphosphate}
oxobutyl] diphosphate}
CAS: Not Available
Description: An acyl-CoA oxoanion that is the pentaanion of hexadecanedioyl-CoA, arising from deprotonation of the phosphate, diphosphate and carboxylic acid functions; major species at pH 7.3.
glucosyl-(heptosyl)2-Kdo2-lipid A-phosphate (PAMDB120526)
IUPAC:
Not Available
CAS: Not Available
Description: A lipid A oxoanion obtained via deprotonation of the carboxy and phosphate OH groups of glucosyl-heptosyl-4-phosphoheptosyl-(KDO)2-lipid A
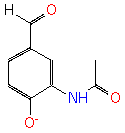
3-acetylamino-4-hydroxybenzaldehyde (PAMDB120527)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
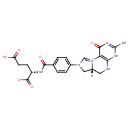
5,10-methenyltetrahydrofolate (PAMDB120528)
IUPAC:
N- {4-
{4- [(6aR)-
[(6aR)- 3-
3- amino-
amino- 1-
1- oxo-
oxo- 1,2,5,6,6a,7-
1,2,5,6,6a,7- hexahydro-
hexahydro- 8H-
8H- imidazo[1,5-
imidazo[1,5- f]pteridin-
f]pteridin- 10-
10- ium-
ium- 8-
8- yl]benzoyl}-
yl]benzoyl}- L-
L- glutamate
glutamate
CAS: 7444-29-3
Description: A dicarboxylic acid anion arising from deprotonation of both carboxylic acid functions of (6R)-5,10-methenyltetrahydrofolic acid.
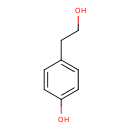
4-tyrosol (PAMDB120529)
IUPAC:
4-(2-hydroxyethyl)phenol
CAS: 501-94-0
Description: A phenol substituted at position 4 by a 2-hydroxyethyl group.