|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120529 |
|---|
|
Identification |
|---|
| Name: |
4-tyrosol |
|---|
| Description: | A phenol substituted at position 4 by a 2-hydroxyethyl group. |
|---|
|
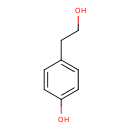
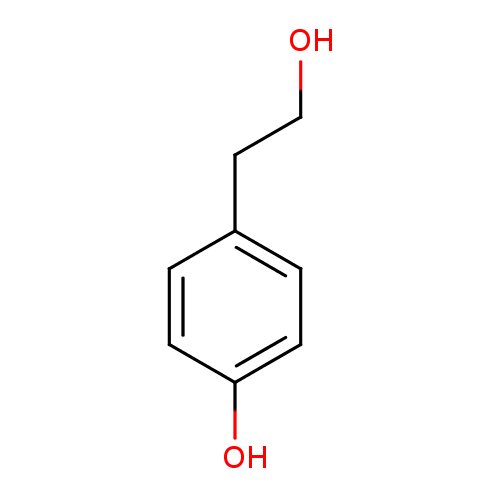
Structure |
|
|---|
| Synonyms: | - 4-Hydroxybenzeneethanol
- 4-Hydroxyphenylethanol
- p-Hydroxyphenethyl alcohol
- tyrosol
- Tyrosol
|
|---|
|
Chemical Formula: |
C8H10O2 |
|---|
| Average Molecular Weight: |
138.166 |
|---|
| Monoisotopic Molecular
Weight: |
138.06808 |
|---|
| InChI Key: |
YCCILVSKPBXVIP-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H10O2/c9-6-5-7-1-3-8(10)4-2-7/h1-4,9-10H,5-6H2 |
|---|
| CAS
number: |
501-94-0 |
|---|
| IUPAC Name: | 4-(2-hydroxyethyl)phenol |
|---|
|
Traditional IUPAC Name: |
tyrosol |
|---|
| SMILES: | C1(C(=CC=C(C=1)O)CCO) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as tyrosols. These are organic aromatic compounds containing a phenethyl alcohol moiety that carries a hydroxyl group at the 4-position of the benzene group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Phenols |
|---|
|
Direct Parent |
Tyrosols |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tyrosol
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
90 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 90 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Peng X, Wang Y, Sun K, Liu P, Yin X, Zhu W (2011)Cerebrosides and 2-pyridone alkaloids from the halotolerant fungus Penicillium chrysogenum grown in a hypersaline medium. Journal of natural products 74, Pubmed: 21381678
- Almeida C, Part N, Bouhired S, Kehraus S, König GM (2011)Stachylines A-D from the sponge-derived fungus Stachylidium sp. Journal of natural products 74, Pubmed: 21162532
|
|---|
| Synthesis Reference: |
Zheng, Hong; Gao, Wenfang; Ji, Xueshi; Zhang, Shoufang. Improved method for synthesis of Tyrosol. Zhongguo Yaowu Huaxue Zazhi (2002), 12(3), 166-167. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|