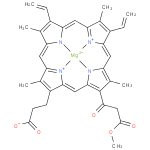
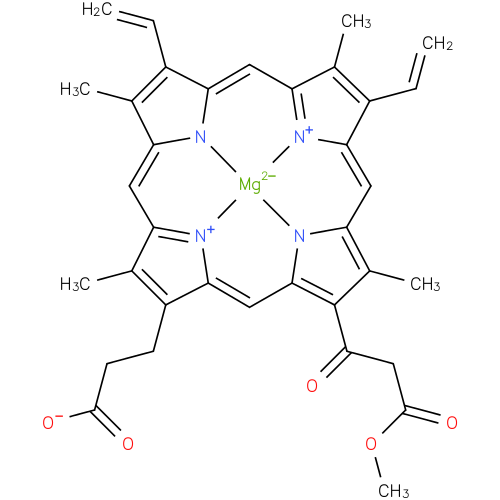
131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester (PAMDB120520)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB120520 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | The conjugate base of magnesium 131-oxoprotoporphyrin 13-monomethyl ester, formed by deprotonation of the carboxyethyl group at C-17. It is the principal species at pH 7.3. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C35H31N4O5MG | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 611.959 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 612.2223 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | IOQIILLGNAOXJE-JXBSUKTBSA-K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C35H34N4O5.Mg/c1-8-21-17(3)24-12-25-19(5)23(10-11-33(41)42)30(38-25)15-31-35(32(40)16-34(43)44-7)20(6)27(39-31)14-29-22(9-2)18(4)26(37-29)13-28(21)36-24;/h8-9,12-15H,1-2,10-11,16H2,3-7H3,(H3,36,37,38,39,40,41,42);/q;+2/p-3/b24-12-,25-12-,26-13-,27-14-,28-13-,29-14-,30-15-,31-15-; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {3- | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C=CC2(C(C)=C6(C=C1(C(C)=C(CCC(=O)[O-])C(=N1)C=C4(N5([Mg]N(C=2C=C3(C(C)=C(C=C)C(=N3)C=C(C(C)=C(C(=O)CC(=O)OC)4)5))6))))) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as metalloporphyrins. These are polycyclic compounds containing a porphyrin moiety and a metal atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Metallotetrapyrroles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Metalloporphyrins | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 131-hydroxy-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ → 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + NADPH + Hydrogen ion 131-hydroxy-magnesium-protoporphyrin IX 13-monomethyl ester + NADPH + Hydrogen ion + Oxygen → 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ + Water 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + Oxygen + NADPH → 2,4-divinyl protochlorophyllide a + NADP+ + Water 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ → 2,4-divinyl protochlorophyllide a + NADPH + Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||