Search Results for compounds
Searching compounds for
returned 4373 results.
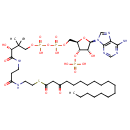
3-Oxooctadecanoyl-CoA (PAMDB001579)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-2,2-dimethyl-3-{[2-({2-[(3-oxooctadecanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 86370-20-9
Description: 3-Oxooctadecanoyl-CoA is a metabolite intermediate in the microsomal fatty acid chain elongation system. Microsomal electron-transport components NADPH-cytochrome P450 reductase (EC 1.6.2.4) and cytochrome b5 (EC 1.6.2.2) participate in the conversion from 3-Oxooctadecanoyl-CoA to beta-hydroxystearoyl-CoA, the first reductive step of the microsomal chain elongating system initiated by NADPH.
3-Oxo-5,6-dehydrosuberyl-CoA (PAMDB001581)
IUPAC:
8-({2-[(3-{[(2R)-4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-1,2-dihydroxy-3,3-dimethylbutylidene]amino}-1-hydroxypropylidene)amino]ethyl}sulfanyl)-6,8-dioxooct-3-enoic acid
CAS: Not Available
Description: 3-Oxo-5,6-dehydrosuberyl-CoA is a member of the chemical class known as Coenzyme A and Derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine.
4-Amino-4-deoxy-L-arabinose modified core oligosaccharide lipid A (PAMDB001582)
IUPAC:
2-{[(3S,4R,5R,6R)-3-({[(5-amino-3,4-dihydroxyoxan-2-yl)oxy](hydroxy)phosphoryl}oxy)-5-{[(3R)-3-(dodecanoyloxy)-1-oxidotetradecylidene]amino}-6-{[(2R,3S,4R,5R)-3-hydroxy-5-{[(3R)-3-hydroxy-1-oxidotetradecylidene]amino}-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}-4-[(2-carboxylato-4-{[2-carboxylato-6-(1,2-dihydroxyethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-(1,2-dihydroxyethyl)-5-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl)oxy]-6-(1,2-dihydroxyethyl)-5-{[6-(1,2-dihydroxyethyl)-4-{[6-(2-{[6-(1,2-dihydroxyethyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-1-hydroxyethyl)-4-({4-[(3-{[6-({[6-(1,2-dihydroxyethyl)-3,4,5-trihydroxyoxan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl)oxy]-3,5-dihydroxy-6-({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl}oxy)-3-hydroxy-5-(phosphonatooxy)oxan-2-yl]oxy}-3-hydroxy-5-(phosphonatooxy)oxan-2-yl]oxy}oxane-2-carboxylate
CAS: Not Available
Description: 4-amino-4-deoxy-l-arabinose modified core oligosaccharide lipid a belongs to the class of Polyhexoses. These are polysaccharides in which the saccharide units are hexoses. (inferred from compound structure)
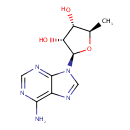
5'-Deoxyadenosine (PAMDB001583)
IUPAC:
(2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-methyloxolane-3,4-diol
CAS: 4754-39-6
Description: 5'-Deoxyadenosine is an abnormal form of deoxyadenosine derived from S-adenosylmethionine. The normal form of deoxyadenosine used in DNA synthesis and repair is 2'-deoxyadenosine where the hydroxyl group (-OH) is at the 2' position of its ribose sugar moiety. 5'-deoxyadenosine has its hydroxyl group at the 5' position of the ribose sugar. 5'-deoxyadenosine is a substrate for 5'-methylthioadenosine phosphorylase. It is also a product of the degradation/conversion of S-adenosyl-methionine by the enzymes: 2-iminoacetate synthase, biotin synthase, lopoyl synthase and pyruvate-formate lyase activating enzyme
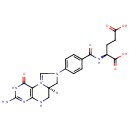
5,10-Methenyltetrahydrofolate (PAMDB001585)
IUPAC:
(6aR)-3-amino-8-(4-{[(1S)-1,3-dicarboxypropyl]carbamoyl}phenyl)-1-oxo-1H,2H,5H,6H,6aH,7H,8H-10???imidazo[1,5-f]pteridin-10-ylium
CAS: 7444-29-3
Description: 5,10-Methenyltetrahydrofolate (5,10-CH=THF) is a form of tetrahydrofolate that is an intermediate in metabolism. 5,10-CH=THF is a coenzyme that accepts and donates methenyl (CH=) groups.;Methylene tetrahydrofolate (CH2FH4) is formed from tetrahydrofolate by the addition of methylene groups from one of three carbon donors: formaldehyde, serine, or glycine. Methyl tetrahydrofolate(CH3FH4) can be made from methylene tetrahydrofolate by reduction of the methylene group, and formyl tetrahydrofolate (CHOFH4, folinic acid) is made by oxidation of methylene tetrahydrofolate.; In the form of a series of tetrahydrofolate compounds, folate derivatives are substrates in a number of single-carbon-transfer reactions, and also are involved in the synthesis of dTMP (2'-deoxythymidine-5'-phosphate) from dUMP (2'-deoxyuridine-5'-phosphate).
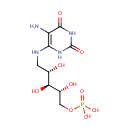
5-Amino-6-(5'-phosphoribitylamino)uracil (PAMDB001586)
IUPAC:
{[(2R,3S,4S)-5-[(5-amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)amino]-2,3,4-trihydroxypentyl]oxy}phosphonic acid
CAS: Not Available
Description: 5-Amino-6-(5'-phosphoribitylamino)uracil is an intermediate in Riboflavin metabolism. 5-Amino-6-(5'-phosphoribitylamino)uracil is the; 3rd to last step in the synthesis of 7-Hydroxy-6-methyl-8-ribityl lumazine and is converted from 5-Amino-6-(5'-phosphoribosylamino)uracil via the enzyme 5-amino-6-(5-phosphoribosylamino)uracil reductase (EC 1.1.1.193). It is then; converted to 4-(1-D-Ribitylamino)-5-amino-2,6-dihydroxypyrimidine via the enzyme Hydrolases (EC 3.1.3.- ).
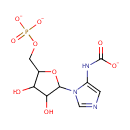
5-Phosphoribosyl-5-carboxyaminoimidazole (PAMDB001587)
IUPAC:
N-(1-{3,4-dihydroxy-5-[(phosphonatooxy)methyl]oxolan-2-yl}-1H-imidazol-5-yl)carbamate
CAS: Not Available
Description: 5-carboxyamino-1-(5-phospho-D-ribosyl)imidazole is an intermediate in purine metabolism and IMP biosynthesis via the de novo pathway. It is a substrate of the PurK enzyme which catalyzes the ATP-dependent conversion of 5-aminoimidazole ribonucleotide (AIR) and HCO3- to N5-carboxyaminoimidazole ribonucleotide (N5-CAIR).
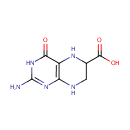
6-Carboxy-5,6,7,8-tetrahydropterin (PAMDB001589)
IUPAC:
2-amino-4-oxo-3,4,5,6,7,8-hexahydropteridine-6-carboxylic acid
CAS: Not Available
Description: 6-carboxy-5,6,7,8-tetrahydropterin is a member of the chemical class known as Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon).6-carboxy-5,6,7,8-tetrahydropterin is catalyzed by QueD. Pseudomonas aeruginosa QueD is a 6-carboxy-5,6,7,8-tetrahydropterin synthase. (PMID 19231875)
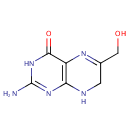
6-Hydroxymethyl dihydropterin (PAMDB001590)
IUPAC:
2-amino-6-(hydroxymethyl)-3,4,7,8-tetrahydropteridin-4-one
CAS: Not Available
Description: 6-hydroxymethyl dihydropterin is a member of the chemical class known as Pterins and Derivatives. These are polycyclic aromatic compounds containing a pterin moeity, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one.
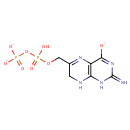
6-Hydroxymethyl-dihydropterin pyrophosphate (PAMDB001591)
IUPAC:
6-({[hydroxy(phosphonatooxy)phosphoryl]oxy}methyl)-2-imino-1,2,7,8-tetrahydropteridin-4-olate
CAS: Not Available
Description: 6-hydroxymethyl-dihydropterin pyrophosphate is a member of the chemical class known as Pterins and Derivatives. These are polycyclic aromatic compounds containing a pterin moeity, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one.