|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001586 |
|---|
|
Identification |
|---|
| Name: |
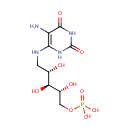
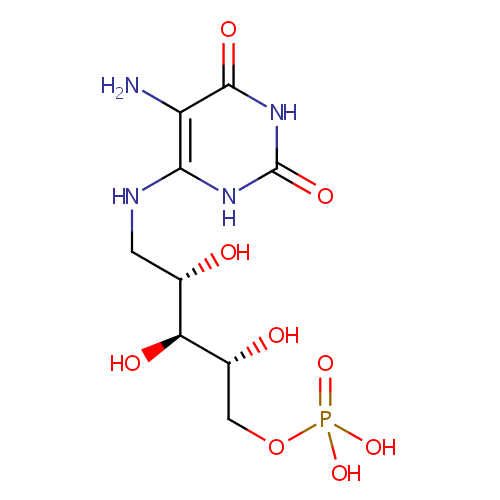
5-Amino-6-(5'-phosphoribitylamino)uracil |
|---|
| Description: | 5-Amino-6-(5'-phosphoribitylamino)uracil is an intermediate in Riboflavin metabolism. 5-Amino-6-(5'-phosphoribitylamino)uracil is the; 3rd to last step in the synthesis of 7-Hydroxy-6-methyl-8-ribityl lumazine and is converted from 5-Amino-6-(5'-phosphoribosylamino)uracil via the enzyme 5-amino-6-(5-phosphoribosylamino)uracil reductase (EC 1.1.1.193). It is then; converted to 4-(1-D-Ribitylamino)-5-amino-2,6-dihydroxypyrimidine via the enzyme Hydrolases (EC 3.1.3.- ). |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1-(5-Amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-ylamino)-1-deoxy-5-O-phosphono-D-ribitol
- 1-(5-Amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-ylamino)-1-deoxy-D-ribitol 5-(dihydrogen phosphate)
- 1-(5-amino-2,6-dioxo-1,2,3,6-Tetrahydropyrimidin-4-ylamino)-1-deoxy-D-ribitol 5-(dihydrogen phosphoric acid)
- 1-[(5-Amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)amino]-1-deoxy-5-O-phosphono-D-ribitol
- 5-Amino-2,6-dioxy-4-(5'-phospho-D-ribitylamino)pyrimidine
- 5-Amino-2,6-dioxy-4-(5'-phosphoribitylamino)pyrimidine
- 5-Amino-6-(5'-phospho-D-ribitylamino)uracil
- 5-Amino-6-(5'-phosphoribitylamino)uracil
- 5-Amino-6-(5-phospho-D-ribitylamino)uracil
- 5-Amino-6-(5-phosphoribitylamino)uracil
- 5-Amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5'-phosphate
- 5-amino-6-ribitylamino-2,4(1H,3H)-Pyrimidinedione 5'-phosphoric acid
- 5A6-5'PRbAU
|
|---|
|
Chemical Formula: |
C9H17N4O9P |
|---|
| Average Molecular Weight: |
356.2264 |
|---|
| Monoisotopic Molecular
Weight: |
356.073314674 |
|---|
| InChI Key: |
RQRINYISXYAZKL-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H17N4O9P/c10-5-7(12-9(18)13-8(5)17)11-1-3(14)6(16)4(15)2-22-23(19,20)21/h3-4,6,14-16H,1-2,10H2,(H2,19,20,21)(H3,11,12,13,17,18) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | {[(2R,3S,4S)-5-[(5-amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)amino]-2,3,4-trihydroxypentyl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4S)-5-[(5-amino-2,6-dioxo-1,3-dihydropyrimidin-4-yl)amino]-2,3,4-trihydroxypentyl]oxyphosphonic acid |
|---|
| SMILES: | NC1=C(NCC(O)C(O)C(O)COP(O)(O)=O)NC(=O)NC1=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxypyrimidines. These are organic compounds containing a hydroxyl group attached to a pyrimidine ring. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
|
Direct Parent |
Hydroxypyrimidines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxypyrimidine
- Monoalkyl phosphate
- Secondary aliphatic/aromatic amine
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Heteroaromatic compound
- Secondary alcohol
- Polyol
- 1,2-diol
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|