|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001585 |
|---|
|
Identification |
|---|
| Name: |
5,10-Methenyltetrahydrofolate |
|---|
| Description: | 5,10-Methenyltetrahydrofolate (5,10-CH=THF) is a form of tetrahydrofolate that is an intermediate in metabolism. 5,10-CH=THF is a coenzyme that accepts and donates methenyl (CH=) groups.;Methylene tetrahydrofolate (CH2FH4) is formed from tetrahydrofolate by the addition of methylene groups from one of three carbon donors: formaldehyde, serine, or glycine. Methyl tetrahydrofolate(CH3FH4) can be made from methylene tetrahydrofolate by reduction of the methylene group, and formyl tetrahydrofolate (CHOFH4, folinic acid) is made by oxidation of methylene tetrahydrofolate.; In the form of a series of tetrahydrofolate compounds, folate derivatives are substrates in a number of single-carbon-transfer reactions, and also are involved in the synthesis of dTMP (2'-deoxythymidine-5'-phosphate) from dUMP (2'-deoxyuridine-5'-phosphate). |
|---|
|
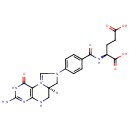
Structure |
|
|---|
| Synonyms: | - (6R)-5,10-CH+-H4folate
- (6R)-5,10-CH+-H4folic acid
- (6R)-5,10-methenyltetrahydrofolate
- (6R)-5,10-methenyltetrahydrofolic acid
- 5,10-Methenyl-THF
- 5,10-Methenyltetrahydrofolate
- 5,10-Methenyltetrahydrofolic acid
- 5,10-Methenyltetrahydropteroylglutamate
- 5,10-Methenyltetrahydropteroylglutamic acid
- 5,10Me-THF
- Anhydro-leucovorin
- Anhydro-leucovorin a
- Anhydroleucovorin
- Anhydroleucovorin a
- Ch-thf
- Methenyl-H4F
- Methenyl-H4F
- Methenyl-tetrahydrofolate
- Methenyl-tetrahydrofolic acid
- Methenyl-thf
- Methenyltetrahydrofolate
- Methenyltetrahydrofolic acid
- N-{4-[(6aR)-3-amino-1-oxo-1,2,5,6,6a,7-hexahydro-8H-imidazo[1,5-f]pteridin-10-ium-8-yl]benzoyl}-L-glutamate
- N-{4-[(6aR)-3-amino-1-oxo-1,2,5,6,6a,7-hexahydro-8H-imidazo[1,5-f]pteridin-10-ium-8-yl]benzoyl}-L-glutamic acid
- N5,n10-methenyl-5,6,7,8-tetrahydrofolate
- N5,n10-methenyl-5,6,7,8-tetrahydrofolic acid
- N5,n10-methenyl-tetrahydrofolate
- N5,n10-methenyl-tetrahydrofolic acid
- N5-n10-ch-thf
- N5-n10-methenyltetrahydrofolate
- N5-n10-methenyltetrahydrofolic acid
|
|---|
|
Chemical Formula: |
C20H22N7O6 |
|---|
| Average Molecular Weight: |
456.432 |
|---|
| Monoisotopic Molecular
Weight: |
456.163156471 |
|---|
| InChI Key: |
MEANFMOQMXYMCT-OLZOCXBDSA-O |
|---|
| InChI: | InChI=1S/C20H21N7O6/c21-20-24-16-15(18(31)25-20)27-9-26(8-12(27)7-22-16)11-3-1-10(2-4-11)17(30)23-13(19(32)33)5-6-14(28)29/h1-4,9,12-13H,5-8H2,(H6-,21,22,23,24,25,28,29,30,31,32,33)/p+1/t12-,13+/m1/s1 |
|---|
| CAS
number: |
7444-29-3 |
|---|
| IUPAC Name: | (6aR)-3-amino-8-(4-{[(1S)-1,3-dicarboxypropyl]carbamoyl}phenyl)-1-oxo-1H,2H,5H,6H,6aH,7H,8H-10???imidazo[1,5-f]pteridin-10-ylium |
|---|
|
Traditional IUPAC Name: |
anhydroleucovorin |
|---|
| SMILES: | [H][C@@]12CN(C=[N+]1C1=C(NC2)N=C(N)NC1=O)C1=CC=C(C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl-alpha amino acids. These are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
N-acyl-alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-alpha-amino acid
- Pterin
- Pteridine
- Imidazopyrazine
- Hydroxypyrimidine
- Secondary aliphatic/aromatic amine
- Benzenoid
- Pyrimidine
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Heteroaromatic compound
- 2-imidazoline
- Tertiary amine
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Carboxylic acid
- Carboximidic acid derivative
- Carboximidic acid
- Carboxylic acid amidine
- Amidine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | Water + 5,10-Methenyltetrahydrofolate <> N10-Formyl-THF + Hydrogen ion5,10-Methylene-THF + NADP <> 5,10-Methenyltetrahydrofolate + NADPH + Hydrogen ionWater + 5,10-Methenyltetrahydrofolate > N5-Formyl-H4F + Hydrogen ion5,10-Methylene-THF + NADP <> 5,10-Methenyltetrahydrofolate + NADPHN5-Formyl-H4F <> 5,10-Methenyltetrahydrofolate + WaterGlycineamideribotide + 5,10-Methenyltetrahydrofolate + Water <> 5'-Phosphoribosyl-N-formylglycineamide + Tetrahydrofolic acidN5-Formyl-H4F + Adenosine triphosphate > 5,10-Methenyltetrahydrofolate + ADP + Phosphate5,10-Methenyltetrahydrofolate + Water > N10-Formyl-THF |
|---|
|
Pathways: |
- Glyoxylate and dicarboxylate metabolism pae00630
- Metabolic pathways pae01100
- Microbial metabolism in diverse environments pae01120
- One carbon pool by folate pae00670
- Reductive carboxylate cycle (CO2 fixation) pae00720
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|