|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001591 |
|---|
|
Identification |
|---|
| Name: |
6-Hydroxymethyl-dihydropterin pyrophosphate |
|---|
| Description: | 6-hydroxymethyl-dihydropterin pyrophosphate is a member of the chemical class known as Pterins and Derivatives. These are polycyclic aromatic compounds containing a pterin moeity, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. |
|---|
|
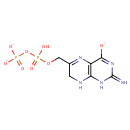
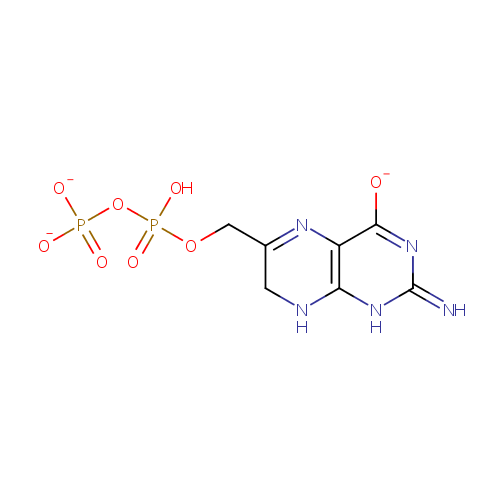
Structure |
|
|---|
| Synonyms: | - (2-amino-4-hydroxy-7,8-dihydropteridin-6-yl)methyl diphosphate
- (2-amino-4-Hydroxy-7,8-dihydropteridin-6-yl)methyl diphosphoric acid
- 2-Amino-7,8-dihydro-4-hydroxy-6-(diphosphooxymethyl)-pteridine
- 6-hydroxymethyl-dihydropterin pyrophosphate
- 6-Hydroxymethyl-dihydropterin pyrophosphoric acid
- AHHMeDHPDP
- Dihydropterin-CH2OH-diphosphate
- Dihydropterin-CH2OH-diphosphoric acid
- Dihydropterin-CH2OH-pp
|
|---|
|
Chemical Formula: |
C7H8N5O8P2 |
|---|
| Average Molecular Weight: |
352.1146 |
|---|
| Monoisotopic Molecular
Weight: |
351.984810281 |
|---|
| InChI Key: |
FCQGJGLSOWZZON-UHFFFAOYSA-K |
|---|
| InChI: | InChI=1S/C7H11N5O8P2/c8-7-11-5-4(6(13)12-7)10-3(1-9-5)2-19-22(17,18)20-21(14,15)16/h1-2H2,(H,17,18)(H2,14,15,16)(H4,8,9,11,12,13)/p-3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-({[hydroxy(phosphonatooxy)phosphoryl]oxy}methyl)-2-imino-1,2,7,8-tetrahydropteridin-4-olate |
|---|
|
Traditional IUPAC Name: |
6-({[hydroxy(phosphonatooxy)phosphoryl]oxy}methyl)-2-imino-7,8-dihydro-1H-pteridin-4-olate |
|---|
| SMILES: | OP(=O)(OCC1=NC2=C(NC1)NC(=N)N=C2[O-])OP([O-])([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pterins and derivatives. These are polycyclic aromatic compounds containing a pterin moiety, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
|
Direct Parent |
Pterins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pterin
- Organic pyrophosphate
- Hydroxypyrimidine
- Secondary aliphatic/aromatic amine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Heteroaromatic compound
- Ketimine
- Azacycle
- Secondary amine
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Imine
- Amine
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|