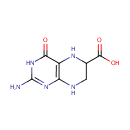
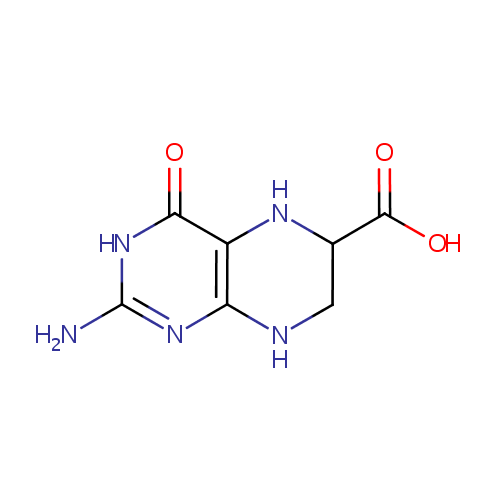
6-Carboxy-5,6,7,8-tetrahydropterin (PAMDB001589)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001589 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 6-Carboxy-5,6,7,8-tetrahydropterin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 6-carboxy-5,6,7,8-tetrahydropterin is a member of the chemical class known as Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon).6-carboxy-5,6,7,8-tetrahydropterin is catalyzed by QueD. Pseudomonas aeruginosa QueD is a 6-carboxy-5,6,7,8-tetrahydropterin synthase. (PMID 19231875) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C7H9N5O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 211.1781 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 211.070539179 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QSIYONWVWDSRRO-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C7H9N5O3/c8-7-11-4-3(5(13)12-7)10-2(1-9-4)6(14)15/h2,10H,1H2,(H,14,15)(H4,8,9,11,12,13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-amino-4-oxo-3,4,5,6,7,8-hexahydropteridine-6-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 2-amino-4-oxo-5,6,7,8-tetrahydro-3H-pteridine-6-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC2=C(NC(CN2)C(O)=O)C(=O)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pterin carboxylates. These are heterocyclic aromatic compounds containing a pterin moiety, in which one ring is substituted by one or more carboxylic acid groups. Pterin is a heterocyclic compound composed of a pteridine ring system (made up of a pyrazine fused to a pyrimidine), with a keto group and an amino group at the 2- and 4- positions, respectively. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pteridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pterins and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pterin carboxylates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Dihydroneopterin triphosphate + Water > Acetaldehyde + 6-Carboxy-5,6,7,8-tetrahydropterin + Hydrogen ion + Triphosphate 6-Carboxy-5,6,7,8-tetrahydropterin <> 7-Carboxy-7-carbaguanine + Ammonia + 7-Deaza-7-carboxyguanine 6-Carboxy-5,6,7,8-tetrahydropterin + S-Adenosylmethionine + Hydrogen ion > 7-carboxy-7-deazaguanine + 5'-Deoxyadenosine + L-Methionine + Ammonia Dihydroneopterin triphosphate + Water > 6-Carboxy-5,6,7,8-tetrahydropterin + Acetaldehyde + Triphosphate 6-Carboxy-5,6,7,8-tetrahydropterin > 7-Deaza-7-carboxyguanine + Ammonia 7,8-dihydroneopterin 3'-triphosphate + Water > Acetaldehyde + Triphosphate +2 Hydrogen ion + 6-Carboxy-5,6,7,8-tetrahydropterin + Triphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Coenzyme transport and metabolism
- Specific function:
- Catalyzes the conversion of 7,8-dihydroneopterin triphosphate (H2NTP) to 6-carboxy-5,6,7,8-tetrahydropterin (CPH4) and acetaldehyde. Can also convert 6-pyruvoyltetrahydropterin (PPH4) and sepiapterin to CPH4; these 2 compounds are probably intermediates in the reaction from H2NTP
- Gene Name:
- queD
- Locus Tag:
- PA2666
- Molecular weight:
- 13.8 kDa
Reactions
| 7,8-dihydroneopterin 3'-triphosphate + H(2)O = 6-carboxy-5,6,7,8-tetrahydropterin + acetaldehyde + triphosphate. |