Search Results for compounds
Searching compounds for
returned 4373 results.
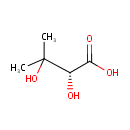
(R)-2,3-Dihydroxy-isovalerate (PAMDB000589)
IUPAC:
(2R)-2,3-dihydroxy-3-methylbutanoic acid
CAS: Not Available
Description: (r)-2,3-dihydroxy-isovalerate belongs to the class of Branched Fatty Acids. These are fatty acids containing a branched chain. (inferred from compound structure)
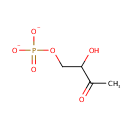
3,4-Dihydroxy-2-butanone-4-P (PAMDB000590)
IUPAC:
3-hydroxy-4-(phosphonatooxy)butan-2-one
CAS: Not Available
Description: 3,4-dihydroxy-2-butanone-4-p is a member of the chemical class known as Organophosphate Esters. These are organic compounds containing phosphoric acid ester functional group. 3,4-dihydroxy-2-butanone-4-p is invovled in Fravin biosynthesis. r 15;80(8):2939-48.)
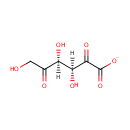
2,5-Diketo-D-gluconate (PAMDB000592)
IUPAC:
(3S,4S)-3,4,6-trihydroxy-2,5-dioxohexanoate
CAS: 53736-12-2
Description: 2,5-Diketo-D-gluconate is an intermediate in the synthesis of ascorbic acid. It is a substrate for the enzyme 2,5-diketo-D-gluconic acid reductase B. This enzyme catalyzes the reduction of 2,5-diketo-D-gluconic acid (25DKG) to 2-keto-L-gulonic acid (2KLG) with the following reaction: 2-dehydro-D-gluconate + NADP+ = 2,5-didehydro-D-gluconate + NADPH.
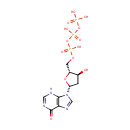
2'-Deoxyinosine triphosphate (PAMDB000593)
IUPAC:
{[hydroxy({[hydroxy({[(2R,3S,5R)-3-hydroxy-5-(6-oxo-6,9-dihydro-3H-purin-9-yl)oxolan-2-yl]methoxy})phosphoryl]oxy})phosphoryl]oxy}phosphonic acid
CAS: 16595-02-1
Description: 2'-Deoxyinosine triphosphate (dITP) is a deoxyribonucleotide that may be generated from dATP by slow, non-enzymatic hydrolysis or by reduction of ITP. Normally, the cellular dITP concentration is very low. The inability to demonstrate the synthesis of dITP in cellular preparations has been attributed to the presence in the cytoplasm of an inosine triphosphatase pyrophosphatase (ITPase, EC 3.6.1.19), an enzyme that does not permit accumulation of these nucleotides. dITP can be incorporated into DNA by polymerases. The deoxyribonucleotide dITP behaves as a dGTP analogue and is incorporated opposite cytosine with about 50% efficiency. Both isolated nuclei and purified DNA polymerases rapidly incorporated dITP into DNA. In the presence of ATP, dITP is stabilized in extracts of nuclei. dITP exist in all cells and is potentially mutagenic, and the levels of these nucleotides are controlled by ITPase. The function of this ubiquitous protein family is proposed to be the elimination of minor potentially mutagenic or clastogenic purine nucleoside triphosphates from the cell.
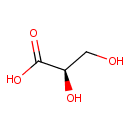
Glyceric acid (PAMDB000597)
IUPAC:
(2R)-2,3-dihydroxypropanoic acid
CAS: 473-81-4
Description: Glyceric acid is a colorless syrupy acid, obtained from oxidation of glycerol. It is a natural three-carbon sugar acid. Salts and esters of glyceric acid are known as glycerates. Several phosphate derivatives of glyceric acid, including 2-phosphoglyceric acid, 3-phosphoglyceric acid, 2,3-bisphosphoglyceric acid, and 1,3-bisphosphoglyceric acid, are important biochemical intermediates. (Wikipedia)
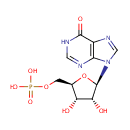
Inosinic acid (PAMDB000601)
IUPAC:
{[(2R,3S,4R,5R)-3,4-dihydroxy-5-(6-oxo-6,9-dihydro-1H-purin-9-yl)oxolan-2-yl]methoxy}phosphonic acid
CAS: 131-99-7
Description: Inosinic acid is a purine nucleotide which has hypoxanthine as the base and one phosphate group esterified to the sugar moiety. It is formed by the deamination of AMP and when hydrolysed produces inosine. Inosinic acid is the ribonucleotide of hypoxanthine and is the first compound formed during the synthesis of purine. (Wikipedia)
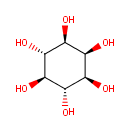
Myoinositol (PAMDB000603)
IUPAC:
(1R,2S,3r,4R,5S,6s)-cyclohexane-1,2,3,4,5,6-hexol
CAS: 87-89-8
Description: Inositol is a cyclic polyalcohol. It exists in nine possible stereoisomers, of which the most prominent form, widely occurring in nature, is cis-1,2,3,5-trans-4,6-cyclohexanehexol, or myo-inositol. It is an isomer of glucose that has traditionally been considered to be a B vitamin although it has an uncertain status as a vitamin. Foods containing the highest concentrations of myo-inositol include fruits, beans, grains and nuts.
Isocitric acid (PAMDB000604)
IUPAC:
1-hydroxypropane-1,2,3-tricarboxylic acid
CAS: 320-77-4
Description: Isocitric acid is a protonated form of isocitrate, which is a substrate of the citric acid cycle. Isocitrate is formed from citrate with the help of the enzyme aconitase, and is acted upon by isocitrate dehydrogenase. Salts and esters of isocitric acid are known as isocitrates. (Wikipedia)
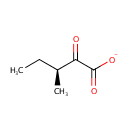
2-Keto-3-methyl-valerate (PAMDB000605)
IUPAC:
(3S)-3-methyl-2-oxopentanoate
CAS: 1460-34-0
Description: 3-Methyl-2-oxovaleric acid is a metabolite of isoleucine in man, animals and bacteria. It is the alpha-keto acid analogue of isoleucine. 3-Methyl-2-oxovaleric acid is produced from isoleucine by cytosolic branched chain aminotransferase 1 (EC:2.6.1.42), whereupon it is further degraded by branched chain keto acid dehydrogenase E1 to 2-Methyl-1-hydroxybutyl-ThPP.
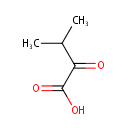
alpha-Ketoisovaleric acid (PAMDB000606)
IUPAC:
3-methyl-2-oxobutanoic acid
CAS: 759-05-7
Description: Alpha-ketoisovaleric acid is a branched chain organic acid which is a precursor to leucine and valine synthesis. It is also a degradation product from valine. The enzyme dihydroxy-acid dehydratase catalyzes the fourth step in the biosynthesis of isoleucine and valine, through the dehydration of 2, 3-dihydroxy-isovaleic acid into alpha-ketoisovaleric acid.