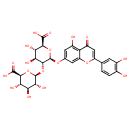Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3801 - 3810 of
4373 in total
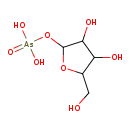
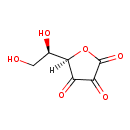
ribose-1-arsenate (PAMDB120060)
IUPAC:
{[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}arsonic acid
CAS: Not Available
Description: Ribose-1-arsenate is an intermediate in arsenate detoxification I pathway. Arsenic detoxification in most mammals involves alternative steps of reduction and oxidative methylation. The end metabolites are methylarsonate,cacodylate, and dimethylarsinous acid,which are less reactive than arsenate and arsenite, and are excreted in the urine.The pathway starts with the reduction of arsenate to arsenite. In this process,arsenate can be conjugate to ribose by the enzyme purine nucleoside phosphorylase (PNP), which accepts arsenate as an alternative substrate to its normal substrate, phosphate. The ribose-1-arsenate thus formed is converted to arsenite in the presence of dihydrolipoate, in a process that has not been fully characterized yet.
5-methyl-3-oxo-4-hexenoyl-CoA (PAMDB120061)
IUPAC:
(2R)-4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-3,3-dimethyl-N-[2-({2-[(5-methyl-3-oxohex-4-enoyl)sulfanyl]ethyl}-C-hydroxycarbonimidoyl)ethyl]butanimidic acid
CAS: Not Available
Description: This compound belongs to the family of Acyl CoAs. These are organic compounds contaning a coenzyme A substructure linked to another moeity through an ester bond.
luteolin 7-O-β-D-diglucuronide (PAMDB120062)
IUPAC:
2- (3,4-
(3,4- dihydroxyphenyl)-
dihydroxyphenyl)- 5-
5- hydroxy-
hydroxy- 4-
4- oxo-
oxo- 4H-
4H- chromen-
chromen- 7-
7- yl (β-
yl (β- D-
D- glucopyranosyluronic acid)-
glucopyranosyluronic acid)- (1→2)-
(1→2)- (β-
(β- D-
D- glucopyranosiduronic acid)
glucopyranosiduronic acid)
CAS: Not Available
Description: This compound belongs to the family of Flavonoid O-Glycosides. These are compounds containing a carbohydrate moiety which is o-glycosidically linked to one of the flavonoid backbones (2-phenylchromen-4-one, 3-phenylchromen-4-one or 4-phenylcoumarin).
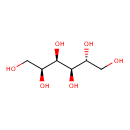
D-sorbitol (PAMDB120063)
IUPAC:
D-glucitol
CAS: 50-70-4
Description: The D-enantiomer of glucitol (also known as D-sorbitol).
L-dehydro-ascorbate (PAMDB120064)
IUPAC:
2-(1,2-dihydroxyethyl)-3,4,5-trioxooxolan-2-ide
CAS: 490-83-5
Description: Conjugate base of dehydroascorbic acid arising from removal of the acidic proton at the C-2 position; major species at pH 7.3.
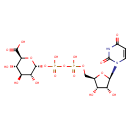
UDP-α-D-glucuronate (PAMDB120066)
IUPAC:
(2S,3S,4S,5R,6R)-6-({[({[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-3,4,5-trihydroxyoxane-2-carboxylic acid
CAS: 2616-64-0
Description: A nucleotide-sugar oxoanion that is a trianion arising from deprotonation of the carboxy and diphosphate OH groups of UDP-α-D-glucuronic acid; major species at pH 7.3.
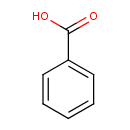
benzoate (PAMDB120067)
IUPAC:
benzoate
CAS: 65-85-0
Description: The simplest member of the class of benzoates that is the conjugate base of benzoic acid, comprising a benzoic acid core with a proton missing to give a charge of -1.
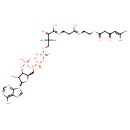
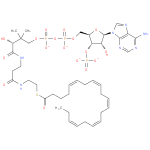
eicosapentaenoyl-CoA (PAMDB120068)
IUPAC:
3'- phosphonatoadenosine 5'-
phosphonatoadenosine 5'- {3-
{3- [(3R)-
[(3R)- 3-
3- hydroxy-
hydroxy- 2,2-
2,2- dimethyl-
dimethyl- 4-
4- oxo-
oxo- 4-
4- ({3-
({3- [(2-
[(2- {[(5Z,8Z,11Z,14Z,17Z)-
{[(5Z,8Z,11Z,14Z,17Z)- icosa-
icosa- 5,8,11,14,17-
5,8,11,14,17- pentaenoyl]sulfanyl}ethyl)amino]-
pentaenoyl]sulfanyl}ethyl)amino]- 3-
3- oxopropyl}amino)butyl] diphosphate}
oxopropyl}amino)butyl] diphosphate}
CAS: Not Available
Description: An acyl-CoA(4−) obtained by deprotonation of the phosphate and diphosphate OH groups of (5Z,8Z,11Z,14Z,17Z)-icosapentaenoyl-CoA.
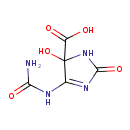
2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline (PAMDB120069)
IUPAC:
4-(carbamoylamino)-5-hydroxy-2-oxo-2,5-dihydro-1H-imidazole-5-carboxylate
CAS: Not Available
Description: Conjugate base of 5-hydroxy-2-oxo-4-ureido-2,5-dihydro-1H-imidazole-5-carboxylic acid.