Record Information Version
1.0 Update Date
1/22/2018 11:54:54 AM
Metabolite ID PAMDB120060
Identification Name:
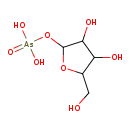
ribose-1-arsenate Description: Ribose-1-arsenate is an intermediate in arsenate detoxification I pathway. Arsenic detoxification in most mammals involves alternative steps of reduction and oxidative methylation. The end metabolites are methylarsonate,cacodylate, and dimethylarsinous acid,which are less reactive than arsenate and arsenite, and are excreted in the urine.The pathway starts with the reduction of arsenate to arsenite. In this process,arsenate can be conjugate to ribose by the enzyme purine nucleoside phosphorylase (PNP), which accepts arsenate as an alternative substrate to its normal substrate, phosphate. The ribose-1-arsenate thus formed is converted to arsenite in the presence of dihydrolipoate, in a process that has not been fully characterized yet.
Structure
Synonyms: Not Available Chemical Formula C5 H11 AsO8 Average Molecular Weight 274.0576 Monoisotopic Molecular Weight 273.966988745 IUPAC Name {[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}arsonic acid Traditional Name [3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxyarsonic acid CAS Registry Number Not Available SMILES OCC1OC(O[As](O)(O)=O)C(O)C1O
InChI Identifier InChI=1S/C5H11AsO8/c7-1-2-3(8)4(9)5(13-2)14-6(10,11)12/h2-5,7-9H,1H2,(H2,10,11,12)
InChI Key RYJJOMQPAAUFBF-UHFFFAOYSA-N Chemical Taxonomy Description This compound belongs to the class of chemical entities known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. Kingdom Chemical entities Super Class Organic compounds Class Organic oxygen compounds Sub Class Organooxygen compounds Direct Parent Pentoses Alternative Parents Substituents Pentose monosaccharide Organic arsenate Tetrahydrofuran Secondary alcohol Oxacycle Organic metalloid salt Organoheterocyclic compound Organic oxide Hydrocarbon derivative Organic salt Primary alcohol Alcohol Aliphatic heteromonocyclic compound Molecular Framework Aliphatic heteromonocyclic compounds External Descriptors Not Available Ontology Status Expected but not Quantified Origin Biofunction Not Available Application Not Available Cellular locations Not Available Physical Properties State Solid Experimental Properties Property Value Reference Melting Point Not Available Not Available Boiling Point Not Available Not Available Water Solubility Not Available Not Available LogP Not Available Not Available
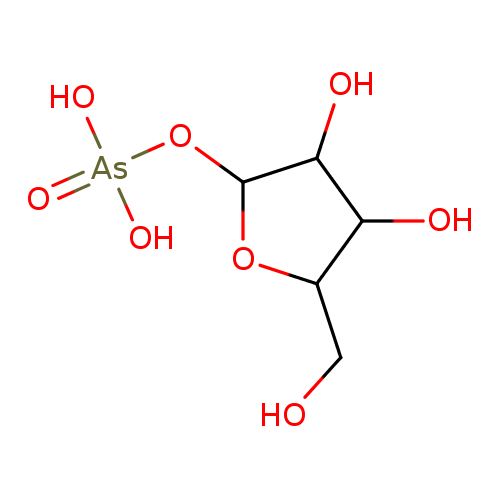
Chemical Formula:
C5 H9 O8 AS Average Molecular Weight:
272.043 Monoisotopic Molecular
Weight:
273.96698 InChI Key:
RYJJOMQPAAUFBF-TXICZTDVSA-L InChI: InChI=1S/C5H11AsO8/c7-1-2-3(8)4(9)5(13-2)14-6(10,11)12/h2-5,7-9H,1H2,(H2,10,11,12)/p-2/t2-,3-,4-,5-/m1/s1 CAS
number:
Not Available IUPAC Name: {[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}arsonic acid
Traditional IUPAC Name:
[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxyarsonic acid SMILES: C(O)C1(C(O)C(O)C(O[As]([O-])(=O)[O-])O1)
Chemical Taxonomy
Taxonomy Description This compound belongs to the class of chemical entities known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms.
Kingdom
Chemical entities Super Class Organic compounds
Class
Organic oxygen compounds Sub Class Organooxygen compounds
Direct Parent
Pentoses Alternative Parents
Substituents
Pentose monosaccharide Organic arsenate Tetrahydrofuran Secondary alcohol Oxacycle Organic metalloid salt Organoheterocyclic compound Organic oxide Hydrocarbon derivative Organic salt Primary alcohol Alcohol Aliphatic heteromonocyclic compound Molecular Framework
Aliphatic heteromonocyclic compounds External Descriptors
Not Available
Physical Properties State:
Solid Charge: Not Available
Melting point:
Not Available Experimental Properties:
Property Value Reference Melting Point Not Available Not Available Boiling Point Not Available Not Available Water Solubility Not Available Not Available LogP Not Available Not Available
Predicted Properties
Biological Properties Cellular Locations:
Not Available Reactions:
Pathways:
Nicotinate and Nicotinamide Metabolism pae00760
Spectra Spectra:
References References:
Not Available Synthesis Reference:
Not Available Material Safety Data Sheet (MSDS)
Not Available
Links External Links:
This project is supported by
the University of Maryland ,
School of Pharmacy ,
Mass Spectrometry Center , a Waters Center of Excellence. The center is an NIH-investigator funded research and core facility that supports a wide range of cutting-edge metabolomic studies. The Center is supported through a center grant from the University of Maryland and NIH grants to its members. The PAMDB project is affiliated with
The Metabolomics Innovation Centre (TMIC) a leading metabolomics research and service center funded through Genome Alberta, Genome British Columbia and Genome Canada.