|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120067 |
|---|
|
Identification |
|---|
| Name: |
benzoate |
|---|
| Description: | The simplest member of the class of benzoates that is the conjugate base of benzoic acid, comprising a benzoic acid core with a proton missing to give a charge of -1. |
|---|
|
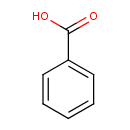
Structure |
|
|---|
| Synonyms: | - Benzenecarboxylate
- Benzeneformate
- Benzenemethanoate
- benzoate
- benzoate anion
- benzoic acid, ion(1−)
- Phenylcarboxylate
- Phenylformate
|
|---|
|
Chemical Formula: |
C7H5O2 |
|---|
| Average Molecular Weight: |
121.115 |
|---|
| Monoisotopic Molecular
Weight: |
122.03678 |
|---|
| InChI Key: |
WPYMKLBDIGXBTP-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)/p-1 |
|---|
| CAS
number: |
65-85-0 |
|---|
| IUPAC Name: | benzoate |
|---|
|
Traditional IUPAC Name: |
benzoic acid |
|---|
| SMILES: | C(C1(C=CC=CC=1))([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as benzoic acids. These are organic Compounds containing a benzene ring which bears at least one carboxyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
|
Direct Parent |
Benzoic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Benzoic acid
- Benzoyl
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
122.4 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 122.4 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 3.4 mg/mL at 25 °C | Not Available | | LogP | 1.87 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0a70-0900000000-5284a0c1c77a1979e1f4 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-056r-3900000000-1c74c32fa650fcd4cb4d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00b9-9500000000-f312a552bef1a2927e64 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004i-9100000000-dafd91c9134bc4143743 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00b9-9400000000-29ca905567aa5c59d46b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7) , Positive | splash10-05i0-6900000000-fa50606b2e84fc4cefe9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6E) , Positive | splash10-0a6r-9600000000-d08dbc757a6de6c3f54e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-7M) , Positive | splash10-0adi-9800000000-2693809ae064e720bf58 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-0pk9-9800000000-2c6be5ecee1848091a24 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00di-0900000000-4644ee08861e75d0b808 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00b9-9400000000-11baabb3c0bb283b1c6e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-004l-9100000000-4875643627420279223b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9000000000-97a21f3206f5c1f0ba7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0pk9-8900000000-3d79c70c455799ab33e3 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Jones MR, Kopple JD, Swendseid ME: Phenylalanine metabolism in uremic and normal man. Kidney Int. 1978 Aug;14(2):169-79. [357810 ]
- Nathan D, Sakr A, Lichtin JL, Bronaugh RL: In vitro skin absorption and metabolism of benzoic acid, p-aminobenzoic acid, and benzocaine in the hairless guinea pig. Pharm Res. 1990 Nov;7(11):1147-51. [2293213 ]
- Downard CD, Roberts LJ 2nd, Morrow JD: Topical benzoic acid induces the increased biosynthesis of prostaglandin D2 in human skin in vivo. Clin Pharmacol Ther. 1995 Apr;57(4):441-5. [7712673 ]
- Temellini A, Mogavero S, Giulianotti PC, Pietrabissa A, Mosca F, Pacifici GM: Conjugation of benzoic acid with glycine in human liver and kidney: a study on the interindividual variability. Xenobiotica. 1993 Dec;23(12):1427-33. [8135043 ]
- Dix KJ, Coleman DP, Jeffcoat AR: Comparative metabolism and disposition of gemfibrozil in male and female Sprague-Dawley rats and Syrian golden hamsters. Drug Metab Dispos. 1999 Jan;27(1):138-46. [9884323 ]
- Parry GE, Bunge AL, Silcox GD, Pershing LK, Pershing DW: Percutaneous absorption of benzoic acid across human skin. I. In vitro experiments and mathematical modeling. Pharm Res. 1990 Mar;7(3):230-6. [2339094 ]
- Nielsen NM, Bundgaard H: Glycolamide esters as biolabile prodrugs of carboxylic acid agents: synthesis, stability, bioconversion, and physicochemical properties. J Pharm Sci. 1988 Apr;77(4):285-98. [3379586 ]
- Killackey JJ, Killackey BA, Philp RB: Cyclic nucleotide phosphodiesterase inhibition by a benzoic acid derivative. Agents Actions. 1985 Dec;17(2):192-6. [2420162 ]
- Nacht S, Yeung D, Beasley JN Jr, Anjo MD, Maibach HI: Benzoyl peroxide: percutaneous penetration and metabolic disposition. J Am Acad Dermatol. 1981 Jan;4(1):31-7. [7204686 ]
- LeBel M, Ferron L, Masson M, Pichette J, Carrier C: Benzyl alcohol metabolism and elimination in neonates. Dev Pharmacol Ther. 1988;11(6):347-56. [3229281 ]
|
|---|
| Synthesis Reference: |
Hronec, Milan; Mikula, Oldrich; Kopernicky, Ivan; Bucko, Milos; Danilla, Frantisek; Hlinistak, Karol. Process for benzoic acid manufacture. Czech. (1987), 3 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|