Search Results for compounds
Searching compounds for
returned 4373 results.
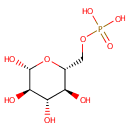
beta-D-Glucose 6-phosphate (PAMDB000506)
IUPAC:
{[(2R,3S,4S,5R,6R)-3,4,5,6-tetrahydroxyoxan-2-yl]methoxy}phosphonic acid
CAS: Not Available
Description: Beta-D-Glucose 6 phosphate (b-G6P) is the beta-anomer of glucose-6-phosphate. There are two anomers of glucose 6 phosphate, the alpha anomer and the beta anomer. Specifically, beta-D-Glucose 6-phosphate is glucose sugar phosphorylated on carbon 6. It is a very common metabolite in cells as the vast majority of glucose entering a cell will become phosphorylated in this way. The primary reason for the immediate phosphorylation of glucose is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane. b-G6P is involved in the glycolysis, gluconeogenesis, pentose phosphate, and glycogen and sucrose metabolic pathways [Kegg ID: C01172]. Beta-D-Glucose 6 phosphate can be generated through beta-D-fructose phosphate or alpha-D-glucose 6 phosphate (via glucose-6-phosphate isomerase) or beta-D glucose (via hexokinase). It can then be sent off to the pentose phosphate pathway which generates the useful cofactor NADPH as well as ribulose 5-phosphate, a carbon source for the synthesis of other molecules. Alternately if the cell needs energy or carbon skeletons for synthesis then glucose 6-phosphate is targeted for glycolysis. A third route is to have glucose 6 phosphate stored or converted to glycogen.
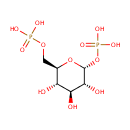
alpha-D-Glucose 1,6-bisphosphate (PAMDB000508)
IUPAC:
{[(2R,3S,4S,5R,6R)-3,4,5-trihydroxy-6-(phosphonooxy)oxan-2-yl]methoxy}phosphonic acid
CAS: 10139-18-1
Description: Alpha-D-glucose 1,6-bisphosphate is considered to be a major regulator of carbohydrate metabolism. Glucose 1,6-diphosphate (G 1,6-P2) have been recognized as a regulatory signal implicated in the control of metabolism, oxygen affinity of red cells and other cellular functions. G 1,6-P2 is a potent allosteric activator of phosphofructokinase.
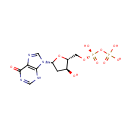
DIDP (PAMDB000509)
IUPAC:
{[hydroxy({[(2R,3S,5R)-3-hydroxy-5-(6-oxo-6,9-dihydro-3H-purin-9-yl)oxolan-2-yl]methoxy})phosphoryl]oxy}phosphonic acid
CAS: 26575-15-5
Description: dIDP (deoxyinoside diphosphate) is a nucleoside. It is an intermediate in purine metabolism. It is converted to dITP or from dITP via multifunctional nucleoside diphosphate kinase and apyrimidinic endonuclease and 3'-phosphodiesterase (EC:2.7.4.6). (KEGG)
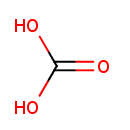
Carbonic acid (PAMDB000510)
IUPAC:
carbonic acid
CAS: 463-79-6
Description: Carbonic acid (ancient name acid of air or aerial acid) is the only inorganic carbon acid, and has the formula H2CO3. It is also a name sometimes given to solutions of carbon dioxide in water, which contain small amounts of H2CO3. The salts of carbonic acids are called bicarbonates (or hydrogencarbonates) and carbonates. (wikipedia)
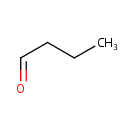
Butanal (PAMDB000512)
IUPAC:
butanal
CAS: 123-72-8
Description: Butanal is an organic compound that is the aldehyde analog of butane. It is a colorless flammable liquid with an acrid smell. It is miscible with most organic solvents.(wikipedia)
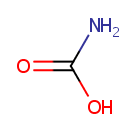
Carbamic acid (PAMDB000513)
IUPAC:
carbamic acid
CAS: 463-77-4
Description: Carbamic acid products of several amines, such as beta-N-methylamino-L-alanine (BMAA), ethylenediamine, and L-cysteine have been implicated in toxicity. Studies suggested that a significant portion of amino-compounds in biological samples (that naturally contain CO2/bicarbonate) can be present as a carbamic acid. There has been no obvious species specificity for their formation and no preference for 1 or 2 degree amines. Many biological reactions have also been described in the literature that involve the reaction of CO2 with amino groups of biomolecules. For example, CO2 generated from cellular respiration is expired in part through the reversible formation of a carbamate between CO2 and the -amino groups of the alpha and beta-chains of hemoglobin. The carbamic acid is also a substrate for glucuronidation and results in a stable carbamate glucuronide metabolite. The detection and characterization of these products has been facilitated greatly by the advent of soft ionization mass spectrometry techniques and high field NMR instrumentation. (PMID: 16268118, 17168688, 12929145)
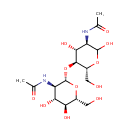
Chitobiose (PAMDB000515)
IUPAC:
N-[(3R,4R,5S,6R)-5-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2,4-dihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide
CAS: 577-76-4
Description: Chitobiose is a dimer of beta-1,4-linked glucosamine units. There is ambiguity as to which structure the name refers, owing to the method by which it was first isolated.
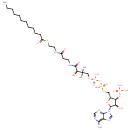
Lauroyl-CoA (PAMDB000516)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-({[({[(3R)-3-[(2-{[2-(dodecanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}methyl)-4-hydroxyoxolan-3-yl]oxy}phosphonic acid
CAS: 6244-92-4
Description: Lauroyl-CoA is a substrate for Long chain fatty acyl-CoA synthetase. This enzyme is a member of the ligase family that activates the breakdown of complex fatty acids. Long chain fatty acyl-CoA synthetase plays a crucial role in intermediary metabolism by catalyzing the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate. It is an enzyme present in all organisms from bacteria to humans.
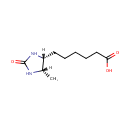
Dethiobiotin (PAMDB000517)
IUPAC:
6-[(4R,5S)-5-methyl-2-oxoimidazolidin-4-yl]hexanoic acid
CAS: 533-48-2
Description: Dethiobiotin is a synthetic metabolite that mimic the effects of biotin on gene expression and thus have biotin-like activities. It is an intermediate in biotin metabolism, and converted to biotin via biotin synthase (EC:2.8.1.6). (KEGG) Biotin serves as a coenzyme for carboxylases such as propionyl-CoA carboxylase. (PMID 12730407)
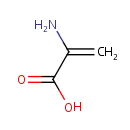
2-Aminoacrylic acid (PAMDB000519)
IUPAC:
2-aminoprop-2-enoic acid
CAS: Not Available
Description: Dehydroalanine (or (alpha)-(beta)-di-dehydroalanine) is an uncommon amino acid found in peptides of microbial origin (an unsaturated amino acid).