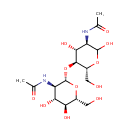
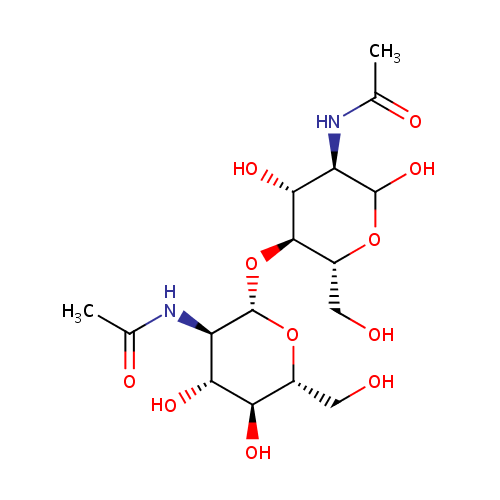
| InChI: | InChI=1S/C16H28N2O11/c1-5(21)17-9-13(25)14(8(4-20)27-15(9)26)29-16-10(18-6(2)22)12(24)11(23)7(3-19)28-16/h7-16,19-20,23-26H,3-4H2,1-2H3,(H,17,21)(H,18,22)/t7-,8-,9-,10-,11-,12-,13-,14-,15?,16+/m1/s1 |
|---|
| References: |
- Collin M, Olsen A: EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001 Jun 15;20(12):3046-55. Pubmed: 11406581
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Nimtz M, Grabenhorst E, Gambert U, Costa J, Wray V, Morr M, Thiem J, Conradt HS: In vitro alpha1-3 or alpha1-4 fucosylation of type I and II oligosaccharides with secreted forms of recombinant human fucosyltransferases III and VI. Glycoconj J. 1998 Sep;15(9):873-83. Pubmed: 10052591
- van Pelt J, Hard K, Kamerling JP, Vliegenthart JF, Reuser AJ, Galjaard H: Isolation and structural characterization of twenty-one sialyloligosaccharides from galactosialidosis urine. An intact N,N'-diacetylchitobiose unit at the reducing end of a diantennary structure. Biol Chem Hoppe Seyler. 1989 Mar;370(3):191-203. Pubmed: 2713102
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|