Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 1081 - 1090 of
4373 in total
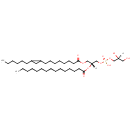
PG(15:0/17:0cycw7c) (PAMDB002031)
IUPAC:
[(2S)-2,3-dihydroxypropoxy][(2R)-3-{[8-(2-hexylcyclopropyl)octanoyl]oxy}-2-(pentadecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PG(15:0/17:0cycw7c) is a phosphatidylglycerol. Phosphatidylglycerols consist of a glycerol 3-phosphate backbone esterified to either saturated or unsaturated fatty acids on carbons 1 and 2. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PG(15:0/17:0cycw7c), in particular, consists of one pentadecanoyl chain to the C-1 atom, and one heptadec-9-10-cyclo-anoyl to the C-2 atom. In Pseudomonas aeruginosa glycerophospholipid metabolism, phosphatidylglycerol is formed from phosphatidic acid (1,2-diacyl-sn-glycerol 3-phosphate) by a sequence of enzymatic reactions that proceeds via two intermediates, cytidine diphosphate diacylglycerol (CDP-diacylglycerol) and phosphatidylglycerophosphate (PGP, a phosphorylated phosphatidylglycerol). Phosphatidylglycerols, along with CDP-diacylglycerol, also serve as precursor molecules for the synthesis of cardiolipin, a phospholipid found in membranes.
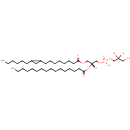
PG(16:0/17:0cycw7c) (PAMDB002032)
IUPAC:
[(2S)-2,3-dihydroxypropoxy][(2R)-2-(hexadecanoyloxy)-3-{[8-(2-hexylcyclopropyl)octanoyl]oxy}propoxy]phosphinic acid
CAS: Not Available
Description: PG(16:0/17:0cycw7c) is a phosphatidylglycerol. Phosphatidylglycerols consist of a glycerol 3-phosphate backbone esterified to either saturated or unsaturated fatty acids on carbons 1 and 2. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PG(16:0/17:0cycw7c), in particular, consists of one hexadecanoyl chain to the C-1 atom, and one heptadec-9-10-cyclo-anoyl to the C-2 atom. In Pseudomonas aeruginosa glycerophospholipid metabolism, phosphatidylglycerol is formed from phosphatidic acid (1,2-diacyl-sn-glycerol 3-phosphate) by a sequence of enzymatic reactions that proceeds via two intermediates, cytidine diphosphate diacylglycerol (CDP-diacylglycerol) and phosphatidylglycerophosphate (PGP, a phosphorylated phosphatidylglycerol). Phosphatidylglycerols, along with CDP-diacylglycerol, also serve as precursor molecules for the synthesis of cardiolipin, a phospholipid found in membranes.
PG(16:0/19:0cycw8c) (PAMDB002033)
IUPAC:
[(2S)-2,3-dihydroxypropoxy][(2R)-3-{[9-(2-heptylcyclopropyl)nonanoyl]oxy}-2-(hexadecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PG(16:0/19:0cycw8c) is a phosphatidylglycerol. Phosphatidylglycerols consist of a glycerol 3-phosphate backbone esterified to either saturated or unsaturated fatty acids on carbons 1 and 2. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PG(16:0/19:0cycw8c), in particular, consists of one hexadecanoyl chain to the C-1 atom, and one 9-(2-heptylcyclopropyl)nonanoyl to the C-2 atom. In Pseudomonas aeruginosa glycerophospholipid metabolism, phosphatidylglycerol is formed from phosphatidic acid (1,2-diacyl-sn-glycerol 3-phosphate) by a sequence of enzymatic reactions that proceeds via two intermediates, cytidine diphosphate diacylglycerol (CDP-diacylglycerol) and phosphatidylglycerophosphate (PGP, a phosphorylated phosphatidylglycerol). Phosphatidylglycerols, along with CDP-diacylglycerol, also serve as precursor molecules for the synthesis of cardiolipin, a phospholipid found in membranes.
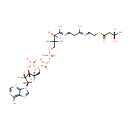
(S)-3-Hydroxybutanoyl-CoA (PAMDB002036)
IUPAC:
(2R)-4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-N-{2-[(2-{[(3S)-3-hydroxybutanoyl]sulfanyl}ethyl)-C-hydroxycarbonimidoyl]ethyl}-3,3-dimethylbutanimidic acid
CAS: Not Available
Description: (S)-3-hydroxybutanoyl-CoA is a member of the chemical class known as Coenzyme A and Derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine.
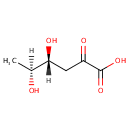
2-Dehydro-3-deoxy-L-rhamnonate (PAMDB002038)
IUPAC:
(4R,5S)-4,5-dihydroxy-2-oxohexanoic acid
CAS: Not Available
Description: 2-dehydro-3-deoxy-l-rhamnonate belongs to the class of Keto Fatty Acids. These are fatty acids containing a ketone group attached to the acyl chain.
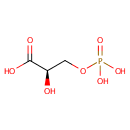
3-Phospho-D-glycerate (PAMDB002039)
IUPAC:
(2R)-2-hydroxy-3-(phosphonooxy)propanoic acid
CAS: Not Available
Description: 3-phospho-D-glycerate is a member of the chemical class known as Sugar Acids and Derivatives. These are compounds containing a saccharide unit which bears a carboxylic acid group.
Aldehyde (PAMDB002040)
IUPAC:
(5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxy-20-oxoicosa-6,8,10,14-tetraenoic acid
CAS: 72379-22-7
Description: A dialdehyde is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending -dial or sometimes -dialdehyde. Short aliphatic dialdehydes are sometimes named after the diacid from which they can de derived. An example is butanedial, which is also called succinaldehyde (from succinic acid).; Aldehydes are readily identified by spectroscopic methods. Using IR spectroscopy, they display a strong ?CO band near 1700 cm??. In their 1H NMR spectra, the formyl hydrogen center absorbs near δ9, which is a distinctive part of the spectrum. This signal shows the characteristic coupling to any protons on the alpha carbon.; An aldehyde ( /??ld?ha?d/) is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center (a carbon double bonded to oxygen) bonded to hydrogen and an R group, which is any generic alkyl or side chain. The group without R is called the aldehyde group or formyl group. Aldehydes differ from ketones in that the carbonyl is placed at the end of a carbon skeleton rather than between two carbon atoms. Aldehydes are common in organic chemistry. Many fragrances are aldehydes.
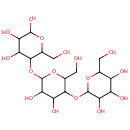
Dextrin (PAMDB002045)
IUPAC:
2-{[4,5-dihydroxy-2-(hydroxymethyl)-6-{[4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}oxan-3-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol
CAS: 9004-53-9
Description: Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch or glycogen. Dextrins are mixtures of polymers of D-glucose units linked by -(1_4) or -(1_6) glycosidic bonds. The cyclical dextrins are known as cyclodextrins. They are formed by enzymatic degradation of starch by certain bacteria, for example, Bacillus macerans. Cyclodextrins have toroidal structures formed by 6-8 glucose residues. (Wikipedia) In Pseudomonas aeruginosa metabolism, the enzyme alpha-amylase (EC 3.2.1.1) catalyzes the breakdown of glycogen into dextrin. (KEGG)
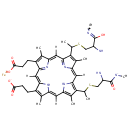
Ferricytochrome c (PAMDB002046)
IUPAC:
??-iron(2+) ion 3-[15-(1-{[2-amino-2-(methyl-C-hydroxycarbonimidoyl)ethyl]sulfanyl}ethyl)-10-(1-{[2-amino-3-(methylazanidyl)-3-oxopropyl]sulfanyl}ethyl)-20-(2-carboxyethyl)-5,9,14,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8,10,12,14,16(22),17,19-undecaen-4-yl]propanoate
CAS: 9007-43-6
Description: Cytochrome c, or Cyt c, is a small heme protein and a component of the oxidative phosphorylation electron transport chain. The heme group of cytochrome c accepts electrons from the Cytochrome b-c1 complex (Complex III) and transfers electrons to the Cytochrome oxidase complex (Complex IV). Cyt c is capable of undergoing oxidation and reduction, but does not bind oxygen. Cytochrome c is a highly conserved protein across the spectrum of species, found in plants, animals, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladistics. Its primary structure consists of a chain of about 100 amino acids. (Wikipedia)
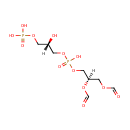
Phosphatidylglycerophosphate (PAMDB002048)
IUPAC:
[(2S)-3-({[(2R)-2,3-bis(formyloxy)propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid
CAS: Not Available
Description: Phosphatidylglycerophosphate belongs to the class of Phosphatidylglycerophosphates. These are glycerophosphoglycerophosphates in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages.